CRISPR/Cas-Based Techniques for Live-Cell Imaging and Bioanalysis
- PMID: 37686249
- PMCID: PMC10487896
- DOI: 10.3390/ijms241713447
CRISPR/Cas-Based Techniques for Live-Cell Imaging and Bioanalysis
Abstract
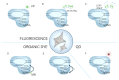
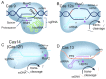
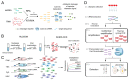
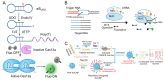
CRISPR/Cas systems have found widespread applications in gene editing due to their high accuracy, high programmability, ease of use, and affordability. Benefiting from the cleavage properties (trans- or cis-) of Cas enzymes, the scope of CRISPR/Cas systems has expanded beyond gene editing and they have been utilized in various fields, particularly in live-cell imaging and bioanalysis. In this review, we summarize some fundamental working mechanisms and concepts of the CRISPR/Cas systems, describe the recent advances and design principles of CRISPR/Cas mediated techniques employed in live-cell imaging and bioanalysis, highlight the main applications in the imaging and biosensing of a wide range of molecular targets, and discuss the challenges and prospects of CRISPR/Cas systems in live-cell imaging and biosensing. By illustrating the imaging and bio-sensing processes, we hope this review will guide the best use of the CRISPR/Cas in imaging and quantifying biological and clinical elements and inspire new ideas for better tool design in live-cell imaging and bioanalysis.
Keywords: CRISPR/Cas; bioanalysis; bioimaging; nucleic acid analysis; protein analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

