Androgens Modulate Bcl-2 Agonist of Cell Death (BAD) Expression and Function in Breast Cancer Cells
- PMID: 37686282
- PMCID: PMC10487823
- DOI: 10.3390/ijms241713464
Androgens Modulate Bcl-2 Agonist of Cell Death (BAD) Expression and Function in Breast Cancer Cells
Abstract
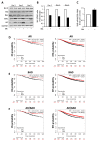
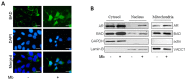
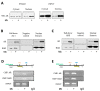
Androgen receptor (AR) expression in estrogen receptor-positive (ER+) breast cancer (BC) correlates with lower tumor grade and a better clinical outcome. Additionally, in normal mammary epithelium or ER+ BC preclinical models, androgens counteract basal/ER-dependent proliferation. Here, we report an additional mechanism, underlining the protective role exerted by AR. Specifically, the activation of intracellular AR upregulates the Bcl-2-family protein BAD, and TCGA database analyses show that in ER+ BC, BAD expression is associated with better disease-free survival. Ligand-activated AR influences its own and BAD cellular compartmentalization by enhancing levels in the nucleus, as well as in mitochondrial fractions. In both compartments, BAD exerts unconventional functions. In the nucleus, BAD and AR physically interact and, upon androgen stimulation, are recruited at the AP-1 and ARE sites within the cyclin D1 promoter region, contributing to explaining the anti-proliferative effect of androgens in BC cells. Androgens cause an enrichment in BAD and AR content in the mitochondria, correlated with a decrease in mitochondrial function. Thus, we have defined a novel mechanism by which androgens modulate BAD expression, its mitochondria localization, and nuclear content to force its ability to act as a cell cycle inhibitor, strengthening the protective role of androgen signaling in estrogen-responsive BCs.
Keywords: AR; BAD; MCF-7; androgens; breast cancer; cyclin D1; mitochondria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
AIB1 sequestration by androgen receptor inhibits estrogen-dependent cyclin D1 expression in breast cancer cells.BMC Cancer. 2019 Nov 4;19(1):1038. doi: 10.1186/s12885-019-6262-4. BMC Cancer. 2019. PMID: 31684907 Free PMC article.
-
Androgen Triggers the Pro-Migratory CXCL12/CXCR4 Axis in AR-Positive Breast Cancer Cell Lines: Underlying Mechanism and Possible Implications for the Use of Aromatase Inhibitors in Breast Cancer.Cell Physiol Biochem. 2017;44(1):66-84. doi: 10.1159/000484584. Epub 2017 Nov 3. Cell Physiol Biochem. 2017. PMID: 29131020
-
The growth response to androgen receptor signaling in ERα-negative human breast cells is dependent on p21 and mediated by MAPK activation.Breast Cancer Res. 2012 Feb 9;14(1):R27. doi: 10.1186/bcr3112. Breast Cancer Res. 2012. PMID: 22321971 Free PMC article.
-
[Dual effects of androgens on mammary gland].Bull Cancer. 2008 May;95(5):495-502. doi: 10.1684/bdc.2008.0631. Bull Cancer. 2008. PMID: 18541513 Review. French.
-
Postmenopausal breast cancer, androgens, and aromatase inhibitors.Breast Cancer Res Treat. 2013 May;139(1):1-11. doi: 10.1007/s10549-013-2505-2. Epub 2013 Apr 10. Breast Cancer Res Treat. 2013. PMID: 23572296 Review.
Cited by
-
Vertical pathway inhibition of receptor tyrosine kinases and BAD with synergistic efficacy in triple negative breast cancer.NPJ Precis Oncol. 2024 Jan 10;8(1):8. doi: 10.1038/s41698-023-00489-3. NPJ Precis Oncol. 2024. PMID: 38200104 Free PMC article.
-
Sex bias in tumor immunity: insights from immune cells.Theranostics. 2025 Mar 31;15(11):5045-5072. doi: 10.7150/thno.106465. eCollection 2025. Theranostics. 2025. PMID: 40303343 Free PMC article. Review.
References
-
- Hammond M.E., Hayes D.F., Dowsett M., Allred D.C., Hagerty K.L., BADve S., Fitzgibbons P.L., Francis G., Goldstein N.S., Hayes M., et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

