Evaluation of the Chemical Stability, Membrane Permeability and Antiproliferative Activity of Cyclic Diarylheptanoids from European Hornbeam (Carpinus betulus L.)
- PMID: 37686297
- PMCID: PMC10488193
- DOI: 10.3390/ijms241713489
Evaluation of the Chemical Stability, Membrane Permeability and Antiproliferative Activity of Cyclic Diarylheptanoids from European Hornbeam (Carpinus betulus L.)
Abstract
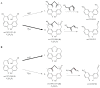
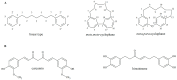
Four cyclic diarylheptanoids-carpinontriols A (1) and B (2), giffonin X (3) and 3,12,17-trihydroxytricyclo [12.3.1.12,6]nonadeca-1(18),2(19),3,5,14,16-hexaene-8,11-dione (4)-were isolated from Carpinus betulus (Betulaceae). Chemical stability of the isolated diarylheptanoids was evaluated as a function of storage temperature (-15, 5, 22 °C) and time (12 and 23 weeks). The effect of the solvent and the pH (1.2, 6.8, 7.4) on the stability of these diarylheptanoids was also investigated. Compounds 2 and 4 showed good stability both in aqueous and methanolic solutions at all investigated temperatures. Only 2 was stable at all three studied biorelevant pH values. Degradation products of 1 and 3 were formed by the elimination of a water molecule from the parent compounds, as confirmed by ultrahigh-performance liquid chromatography-high-resolution tandem mass spectrometry (UHPLC-HR-MS). The permeability of the compounds across biological membranes was evaluated by the parallel artificial membrane permeability assay (PAMPA). Compound 3 possesses a logPe value of -5.92 ± 0.04 in the blood-brain barrier-specific PAMPA-BBB study, indicating that it may be able to cross the blood-brain barrier via passive diffusion. The in vitro antiproliferative activity of the compounds was investigated against five human cancer cell lines, confirming that 1 inhibits cell proliferation in A2058 human metastatic melanoma cells.
Keywords: PAMPA; antiproliferative activity; chemical stability; degradation products; diarylheptanoid; mass spectrometry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
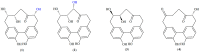
Similar articles
-
Comprehensive Characterization of Phytochemical Composition, Membrane Permeability, and Antiproliferative Activity of Juglans nigra Polyphenols.Int J Mol Sci. 2024 Jun 25;25(13):6930. doi: 10.3390/ijms25136930. Int J Mol Sci. 2024. PMID: 39000038 Free PMC article.
-
Isolation and quantification of diarylheptanoids from European hornbeam (Carpinus betulus L.) and HPLC-ESI-MS/MS characterization of its antioxidative phenolics.J Pharm Biomed Anal. 2022 Feb 20;210:114554. doi: 10.1016/j.jpba.2021.114554. Epub 2021 Dec 27. J Pharm Biomed Anal. 2022. PMID: 34973466
-
Membrane Permeability and Aqueous Stability Study of Linear and Cyclic Diarylheptanoids from Corylus maxima.Pharmaceutics. 2022 Jun 12;14(6):1250. doi: 10.3390/pharmaceutics14061250. Pharmaceutics. 2022. PMID: 35745822 Free PMC article.
-
Recent Studies on Cyclic 1,7-Diarylheptanoids: Their Isolation, Structures, Biological Activities, and Chemical Synthesis.Molecules. 2018 Nov 27;23(12):3107. doi: 10.3390/molecules23123107. Molecules. 2018. PMID: 30486479 Free PMC article. Review.
-
[Advances in parallel artificial membrane permeability assay and its applications].Yao Xue Xue Bao. 2011 Aug;46(8):890-5. Yao Xue Xue Bao. 2011. PMID: 22007511 Review. Chinese.
Cited by
-
Comprehensive Characterization of Phytochemical Composition, Membrane Permeability, and Antiproliferative Activity of Juglans nigra Polyphenols.Int J Mol Sci. 2024 Jun 25;25(13):6930. doi: 10.3390/ijms25136930. Int J Mol Sci. 2024. PMID: 39000038 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- K 120342/National Research, Development and Innovation Office
- FK 125302/National Research, Development and Innovation Office
- K 135712/National Research, Development and Innovation Office
- K-142904/National Research, Development and Innovation Office
- EFOP-1.8.0-VEKOP-17-2017-0000/National Research, Development and Innovation Office
LinkOut - more resources
Full Text Sources

