Mucins 3A and 3B Are Expressed in the Epithelium of Human Large Airway
- PMID: 37686350
- PMCID: PMC10487631
- DOI: 10.3390/ijms241713546
Mucins 3A and 3B Are Expressed in the Epithelium of Human Large Airway
Abstract
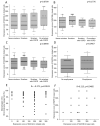
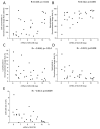
Aberrant mucus secretion is a hallmark of chronic obstructive pulmonary disease (COPD). Expression of the membrane-tethered mucins 3A and 3B (MUC3A, MUC3B) in human lung is largely unknown. In this observational cross-sectional study, we recruited subjects 45-65 years old from the general population of Stockholm, Sweden, during the years 2007-2011. Bronchial mucosal biopsies, bronchial brushings, and bronchoalveolar lavage fluid (BALF) were retrieved from COPD patients (n = 38), healthy never-smokers (n = 40), and smokers with normal lung function (n = 40). Protein expression of MUC3A and MUC3B in bronchial mucosal biopsies was assessed by immunohistochemical staining. In a subgroup of subjects (n = 28), MUC3A and MUC3B mRNAs were quantified in bronchial brushings using microarray. Non-parametric tests were used to perform correlation and group comparison analyses. A value of p < 0.05 was considered statistically significant. MUC3A and MUC3B immunohistochemical expression was localized to ciliated cells. MUC3B was also expressed in basal cells. MUC3A and MUC3B immunohistochemical expression was equal in all study groups but subjects with emphysema had higher MUC3A expression, compared to those without emphysema. Smokers had higher mRNA levels of MUC3A and MUC3B than non-smokers. MUC3A and MUC3B mRNA were higher in male subjects and correlated negatively with expiratory air flows. MUC3B mRNA correlated positively with total cell concentration and macrophage percentage, and negatively with CD4/CD8 T cell ratio in BALF. We concluded that MUC3A and MUC3B in large airways may be a marker of disease or may play a role in the pathophysiology of airway obstruction.
Keywords: COPD; bronchoalveolar lavage; epithelium; immunohistochemistry; large airways; lung function; mRNA; microarray; mucin; smoking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Marshall D.C., Al Omari O., Goodall R., Shalhoub J., Adcock I.M., Chung K.F., Salciccioli J.D. Trends in prevalence, mortality, and disability-adjusted life-years relating to chronic obstructive pulmonary disease in Europe: An observational study of the global burden of disease database, 2001–2019. BMC Pulm. Med. 2022;22:289. - PMC - PubMed
-
- Institute for Health Metrics and Evaluation . Global Burden of Disease Study 2019 (GBD 2019) Results. Institute for Health Metrics and Evaluation; Seattle, WA, USA: 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

