Peri-Operative Kinetics of Plasma Mitochondrial DNA Levels during Living Donor Kidney Transplantation
- PMID: 37686384
- PMCID: PMC10487554
- DOI: 10.3390/ijms241713579
Peri-Operative Kinetics of Plasma Mitochondrial DNA Levels during Living Donor Kidney Transplantation
Abstract
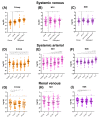
During ischemia and reperfusion injury (IRI), mitochondria may release mitochondrial DNA (mtDNA). mtDNA can serve as a propagator of further injury but in specific settings has anti-inflammatory capacities as well. Therefore, the aim of this study was to study the perioperative dynamics of plasma mtDNA during living donor kidney transplantation (LDKT) and its potential as a marker of graft outcome. Fifty-six donor-recipient couples from the Volatile Anesthetic Protection of Renal Transplants-1 (VAPOR-1) trial were included. Systemic venous, systemic arterial, and renal venous samples were taken at multiple timepoints during and after LDKT. Levels of mtDNA genes changed over time and between vascular compartments. Several donor, recipient, and transplantation-related variables significantly explained the course of mtDNA genes over time. mtDNA genes predicted 1-month and 24-month estimated glomerular filtration rate (eGFR) and acute rejection episodes in the two-year follow-up period. To conclude, mtDNA is released in plasma during the process of LDKT, either from the kidney or from the whole body in response to transplantation. While circulating mtDNA levels positively and negatively predict post-transplantation outcomes, the exact mechanisms and difference between mtDNA genes are not yet understood and need further exploration.
Keywords: DAMPs; kidney transplantation; mtDNA.
Conflict of interest statement
G.J.N.-M.: This author declares no conflict of interest related to this work. In general, her research group has received (over the last 3 years) unrestricted research grants from Astellas Pharma (Leiden, The Netherlands) and Sedana Medical (Danderyd, Sweden). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. M.M.R.F.S.: This author declares no conflict of interest related to this work. In general, his research group/department has received (over the last 3 years) research grants and consultancy fees from Masimo (Irvine, CA, USA), Becton Dickinson (Eysins, Switzerland), Fresenius (Bad Homburg, Germany), Dräger (Lübeck, Germany), Paion (Aachen, Germany), Medcaptain Europe (Andelst, The Netherlands), and Baxter (Chicago, IL, USA). He receives royalties on intellectual property from Demed Medical (Temse, Belgium) and Ghent University (Gent, Belgium). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The other authors declare no conflict of interest.
Figures

References
-
- Abecassis M., Bartlett S.T., Collins A.J., Davis C.L., Delmonico F.L., Friedewald J.J., Hays R., Howard A., Jones E., Leichtman A.B., et al. Kidney transplantation as primary therapy for end-stage renal disease: A National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin. J. Am. Soc. Nephrol. 2008;3:471–480. doi: 10.2215/CJN.05021107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

