A Phase 2, Single-Arm, Open-Label Clinical Trial on Adjuvant Peptide-Based Vaccination in Dogs with Aggressive Hemangiosarcoma Undergoing Surgery and Chemotherapy
- PMID: 37686485
- PMCID: PMC10486958
- DOI: 10.3390/cancers15174209
A Phase 2, Single-Arm, Open-Label Clinical Trial on Adjuvant Peptide-Based Vaccination in Dogs with Aggressive Hemangiosarcoma Undergoing Surgery and Chemotherapy
Abstract
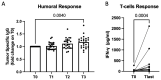
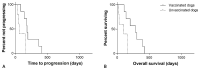
To test the antitumor effect and safety of peptide-based anticancer vaccination in dogs with hemangiosarcoma undergoing the standard of care (SOC; surgery and doxorubicin), canine hemangiosarcoma cells were infected with Salmonella typhi Ty21a to release immunogenic endoplasmic reticulum stress-related peptides into the extracellular milieu via CX43 hemichannels opening. The infected tumor cell secretome constituted the vaccine. Following the SOC, dogs with biologically aggressive hemangiosarcoma were vaccinated a total of five times, once every 3 weeks, and were followed up with serial imaging. A retrospective population of dogs undergoing the SOC alone served as controls. The primary endpoints were the time to progression (TTP) and overall survival (OS), and the secondary endpoints were toxicity and immune responses. A total of 28 dogs were vaccinated along with the SOC, and 32 received only the SOC. A tumor-specific humoral response along with a vaccine-specific T-cell response was observed. Toxicity did not occur. The TTP and OS were significantly longer in vaccinated versus unvaccinated dogs (TTP: 195 vs. 160 days, respectively; p = 0.001; OS: 276 vs. 175 days, respectively; p = 0.002). One-year survival rates were 35.7% and 6.3% for vaccinated and unvaccinated dogs, respectively. In dogs with hemangiosarcoma undergoing the SOC, the addition of a peptide-based vaccine increased the TTP and OS, while maintaining a safe profile. Moreover, vaccinated dogs developed a tumor-specific response, supporting the feasibility of future phase three studies.
Keywords: angiosarcoma; canine; immunotherapy; translational research.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Moore A.S., Rassnick K.M., Frimberger A.E. Evaluation of clinical and histologic factors associated with survival time in dogs with stage II splenic hemangiosarcoma treated by splenectomy and adjuvant chemotherapy: 30 cases (2011–2014) J. Am. Vet. Med. Assoc. 2017;251:559–565. doi: 10.2460/javma.251.5.559. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

