Expression of Stem Cell Markers in High-LET Space Radiation-Induced Intestinal Tumors in Apc1638N/+ Mouse Intestine
- PMID: 37686516
- PMCID: PMC10486545
- DOI: 10.3390/cancers15174240
Expression of Stem Cell Markers in High-LET Space Radiation-Induced Intestinal Tumors in Apc1638N/+ Mouse Intestine
Abstract
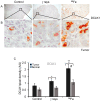
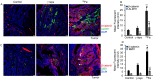
Estimation of cancer risk among astronauts planning to undertake future deep-space missions requires understanding the quantitative and qualitative differences in radiogenic cancers after low- and high-LET radiation exposures. Previously, we reported a multifold higher RBE for high-LET radiation-induced gastrointestinal (GI) tumorigenesis in Apc1638N/+ mice. Using the same model system, i.e., Apc1638N/+ mice, here, we report qualitative differences in the cellular phenotype of low- and high-LET radiation-induced GI tumors. Stem cell (SC) phenotypes were identified using BMI1, ALDH1, CD133, DCLK1, MSI1, and LGR5 markers in low (γ-rays)- and high (56Fe)-LET radiation-induced and spontaneous tumors. We also assessed the expression of these markers in the adjacent normal mucosa. All six of these putative SC markers were shown to be overexpressed in tumors compared to the adjacent normal intestinal tissue. A differential SC phenotype for spontaneous and radiogenic intestinal tumors in Apc1638N/+ mice was observed, where the ALDH1, BMI1, CD133, MSI1, and DCLK1 expressing cells were increased, while LGR5 expressing cells were decreased in 56Fe-induced tumors compared to γ-ray-induced and spontaneous tumors. Furthermore, higher β-catenin activation (marked by nuclear localization) was observed in 56Fe-induced tumors compared to γ and spontaneous tumors. Since differential tumor cell phenotype along with activated β-catenin may very well affect malignant progression, our findings are relevant to understanding the higher carcinogenic risk of high-LET radiation. This study has implications for the assessment of GI-cancer risk among astronauts, as well as for the estimation of secondary cancer risk among patients receiving hadron therapy, considering that our results indicate increased stemness properties after radiation.
Keywords: Apc1638N/+; heavy-ion radiation; intestinal tumor; space radiation; stem cells; β-catenin.
Conflict of interest statement
The authors have no potential conflict of interest relevant to this article. The funders had no role in the in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Rahmanifard F., de Wet W.C., Schwadron N.A., Owens M.J., Jordan A.P., Wilson J.K., Joyce C.J., Spence H.E., Smith C.W., Townsend L.W. Galactic Cosmic Radiation in the Interplanetary Space Through a Modern Secular Minimum. Space Weather. 2020;18:e2019SW002428. doi: 10.1029/2019SW002428. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

