Effective Use of microRNA, BRAF and Sonographic Risk Assessment in Bethesda III Thyroid Nodules Requires a Different Approach to Nodules with Features of Nuclear Atypia and Other Types of Atypia
- PMID: 37686562
- PMCID: PMC10486535
- DOI: 10.3390/cancers15174287
Effective Use of microRNA, BRAF and Sonographic Risk Assessment in Bethesda III Thyroid Nodules Requires a Different Approach to Nodules with Features of Nuclear Atypia and Other Types of Atypia
Abstract
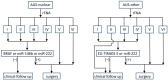
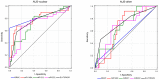
The aim of the study was to analyze the diagnostic usefulness of the combined assessment of the ultrasound risk category of the nodule (evaluated with EU-TIRADS system), the presence of BRAF V600E mutation and the expression of selected microRNAs (miR-146b, miR-221 and miR-222) in Bethesda category III thyroid nodules, separately for cases with nuclear atypia (AUS-nuclear) and cases with other types of atypia (AUS-other). We evaluated 161 nodules (66 AUS-nuclear and 95 AUS-other) with known results of postoperative histopathological examination. The rate of cancer and the rate of PTC among cancers were nearly three times higher in the AUS-nuclear than the AUS-other group. For AUS-nuclear nodules, the most effective diagnostic panel included, in addition to repeat FNA, the assessment of BRAF V600E mutation and the expression of miR-146b and miR-222 (sensitivity: 93.5%, specificity: 80.0%). For AUS-other nodules, a two-step procedure was most effective: at the first stage, forgoing surgical treatment in subjects with a benign repeat FNA outcome, and, at the second stage, the assessment of miR-222 expression and the EU-TIRADS category (sensitivity: 92.3%, specificity: 76.8%). The optimal use of molecular methods in the diagnostics of category III thyroid nodules requires a separate approach for nodules with nuclear atypia and nodules with other types of atypia.
Keywords: AUS-nuclear; AUS-other; BRAF; EU-TIRADS; FNA; microRNA; thyroid cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Haugen B.R., Alexander E.K., Bible K.C., Doherty G.M., Mandel S.J., Nikiforov Y.E., Pacini F., Randolph G.W., Sawka A.M., Schlumberger M., et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

