Positive Regulation of Acetate in Adipocyte Differentiation and Lipid Deposition in Obese Mice
- PMID: 37686768
- PMCID: PMC10489952
- DOI: 10.3390/nu15173736
Positive Regulation of Acetate in Adipocyte Differentiation and Lipid Deposition in Obese Mice
Abstract
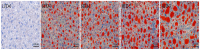
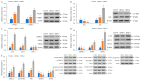
Acetate is associated with adipocyte differentiation and lipid deposition. To further develop this scientific point, obese mice on a high-fat diet were given an intragastric administration of acetate for 8 weeks and mouse adipose mesenchymal stem cells (mAMSCs) were treated with acetate for 24 h. The results showed that the body weight, food intake, Lee's index, adipose tissue coefficient, liver index, blood lipid levels, insulin resistance, pro-inflammatory factors levels and fatty lesions in liver and adipose tissue in obese mice treated with acetate increased markedly, while anti-inflammatory factors levels and liver function decreased significantly (p < 0.05). The mRNA expression levels of PPAR-γ, C/EBP-α, SREBP, AFABP, FAS, ACC-1, SCD-1, LPL, LEPR, GPR41 and GPR43 genes in adipose tissue and mAMSCs were significantly increased, while the mRNA expression levels of HSL, CPT-1, CPT-2, AMPK, AdipoR1 and AdipoR2 genes were significantly reduced (p < 0.05). Except for AMPK-α signaling pathway proteins, the phosphorylation levels of p38 MAPK, ERK1/2, JNK and mTOR were significantly increased (p < 0.05) and these changes were dose-dependent. The findings indicated that acetate played a positive role in regulating adipocyte differentiation and lipid deposition by activating MAPKs and mTOR signaling pathways (the expression up-regulation of genes such as PPAR-γ, C/EBP-α and SREBP-1, etc.) and inhibiting the AMPK signaling pathway (the expression down-regulation of genes such as HSL, CPT-1 and AMPK-α, etc.).
Keywords: acetate; adipocyte differentiation; lipid deposition; lipid metabolism; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Saikosaponin A and D Inhibit Adipogenesis via the AMPK and MAPK Signaling Pathways in 3T3-L1 Adipocytes.Int J Mol Sci. 2021 Oct 22;22(21):11409. doi: 10.3390/ijms222111409. Int J Mol Sci. 2021. PMID: 34768840 Free PMC article.
-
[Punicalagin inhibits hepatic lipid deposition in obese mice via AMPK/ACC pathway].Zhongguo Zhong Yao Za Zhi. 2023 Apr;48(7):1751-1759. doi: 10.19540/j.cnki.cjcmm.20221114.702. Zhongguo Zhong Yao Za Zhi. 2023. PMID: 37282949 Chinese.
-
Acetate alters the process of lipid metabolism in rabbits.Animal. 2018 Sep;12(9):1895-1902. doi: 10.1017/S1751731117003275. Epub 2017 Dec 4. Animal. 2018. PMID: 29198236
-
4-Hydroxyderricin and xanthoangelol from Ashitaba (Angelica keiskei) suppress differentiation of preadiopocytes to adipocytes via AMPK and MAPK pathways.Mol Nutr Food Res. 2013 Oct;57(10):1729-40. doi: 10.1002/mnfr.201300020. Epub 2013 May 16. Mol Nutr Food Res. 2013. PMID: 23681764
-
Novel insights of dietary polyphenols and obesity.J Nutr Biochem. 2014 Jan;25(1):1-18. doi: 10.1016/j.jnutbio.2013.09.001. J Nutr Biochem. 2014. PMID: 24314860 Free PMC article. Review.
Cited by
-
The thrombin receptor PAR4 supports visceral adipose tissue inflammation.Naunyn Schmiedebergs Arch Pharmacol. 2024 Sep;397(9):7187-7200. doi: 10.1007/s00210-024-03097-5. Epub 2024 Apr 23. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38652276 Free PMC article.
-
Glutamate increases the lean percentage and intramuscular fat content and alters gut microbiota in Shaziling pigs.Anim Nutr. 2024 Nov 5;20:110-119. doi: 10.1016/j.aninu.2024.07.010. eCollection 2025 Mar. Anim Nutr. 2024. PMID: 39967691 Free PMC article.
-
Integration of Multi-Omics, Histological, and Biochemical Analysis Reveals the Toxic Responses of Nile Tilapia Liver to Chronic Microcystin-LR Exposure.Toxins (Basel). 2024 Mar 14;16(3):149. doi: 10.3390/toxins16030149. Toxins (Basel). 2024. PMID: 38535815 Free PMC article.
-
Gut-Microbiota-Derived Metabolites and Probiotic Strategies in Colorectal Cancer: Implications for Disease Modulation and Precision Therapy.Nutrients. 2025 Jul 30;17(15):2501. doi: 10.3390/nu17152501. Nutrients. 2025. PMID: 40806086 Free PMC article. Review.
-
Free Fatty Acids and Free Fatty Acid Receptors: Role in Regulating Arterial Function.Int J Mol Sci. 2024 Jul 18;25(14):7853. doi: 10.3390/ijms25147853. Int J Mol Sci. 2024. PMID: 39063095 Free PMC article. Review.
References
-
- Peng W., Zhang J., Zhou H., Zhang A., Wang Y., Tian X., Wen D., Wang Y. Obesity intervention efforts in China and the 2022 World Obesity Day. Glob. Health J. 2022;6:118–121. doi: 10.1016/j.glohj.2022.07.007. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

