Suaeda glauca Attenuates Liver Fibrosis in Mice by Inhibiting TGFβ1-Smad2/3 Signaling in Hepatic Stellate Cells
- PMID: 37686772
- PMCID: PMC10490352
- DOI: 10.3390/nu15173740
Suaeda glauca Attenuates Liver Fibrosis in Mice by Inhibiting TGFβ1-Smad2/3 Signaling in Hepatic Stellate Cells
Abstract
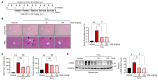
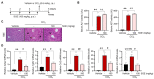
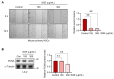
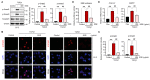
Chronic liver injury due to various hepatotoxic stimuli commonly leads to fibrosis, which is a crucial factor contributing to liver disease-related mortality. Despite the potential benefits of Suaeda glauca (S. glauca) as a natural product, its biological and therapeutic effects are barely known. This study investigated the effects of S. glauca extract (SGE), obtained from a smart farming system utilizing LED lamps, on the activation of hepatic stellate cells (HSCs) and the development of liver fibrosis. C57BL/6 mice received oral administration of either vehicle or SGE (30 or 100 mg/kg) during CCl4 treatment for 6 weeks. The supplementation of SGE significantly reduced liver fibrosis induced by CCl4 in mice as evidenced by histological changes and a decrease in collagen accumulation. SGE treatment also led to a reduction in markers of HSC activation and inflammation as well as an improvement in blood biochemical parameters. Furthermore, SGE administration diminished fibrotic responses following acute liver injury. Mechanistically, SGE treatment prevented HSC activation and inhibited the phosphorylation and nuclear translocation of Smad2/3, which are induced by transforming growth factor (TGF)-β1 in HSCs. Our findings indicate that SGE exhibits anti-fibrotic effects by inhibiting TGFβ1-Smad2/3 signaling in HSCs.
Keywords: Suaeda glauca extract; TGFβ1; hepatic stellate cells; liver fibrosis; smart farming system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

