A High-Fat, High-Cholesterol Diet Promotes Intestinal Inflammation by Exacerbating Gut Microbiome Dysbiosis and Bile Acid Disorders in Cholecystectomy
- PMID: 37686860
- PMCID: PMC10489946
- DOI: 10.3390/nu15173829
A High-Fat, High-Cholesterol Diet Promotes Intestinal Inflammation by Exacerbating Gut Microbiome Dysbiosis and Bile Acid Disorders in Cholecystectomy
Abstract
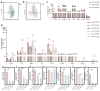
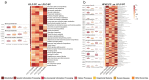
Patients with post-cholecystectomy (PC) often experience adverse gastrointestinal conditions, such as PC syndrome, colorectal cancer (CRC), and non-alcoholic fatty liver disease (NAFLD), that accumulate over time. An epidemiological survey further revealed that the risk of cholecystectomy is associated with high-fat and high-cholesterol (HFHC) dietary intake. Mounting evidence suggests that cholecystectomy is associated with disrupted gut microbial homeostasis and dysregulated bile acids (BAs) metabolism. However, the effect of an HFHC diet on gastrointestinal complications after cholecystectomy has not been elucidated. Here, we aimed to investigate the effect of an HFHC diet after cholecystectomy on the gut microbiota-BA metabolic axis and elucidate the association between this alteration and the development of intestinal inflammation. In this study, a mice cholecystectomy model was established, and the levels of IL-Iβ, TNF-α, and IL-6 in the colon were increased in mice fed an HFHC diet for 6 weeks. Analysis of fecal BA metabolism showed that an HFHC diet after cholecystectomy altered the rhythm of the BA metabolism by upregulating liver CPY7A1, CYP8B1, and BSEP and ileal ASBT mRNA expression levels, resulting in increased fecal BA levels. In addition, feeding an HFHC diet after cholecystectomy caused a significant dysbiosis of the gut microbiota, which was characterized by the enrichment of the metabolic microbiota involved in BAs; the abundance of pro-inflammatory gut microbiota and related pro-inflammatory metabolite levels was also significantly higher. In contrast, the abundance of major short-chain fatty acid (SCFA)-producing bacteria significantly decreased. Overall, our study suggests that an HFHC diet after cholecystectomy promotes intestinal inflammation by exacerbating the gut microbiome and BA metabolism dysbiosis in cholecystectomy. Our study also provides useful insights into the maintenance of intestinal health after cholecystectomy through dietary or probiotic intervention strategies.
Keywords: bile acids; cholesterol; diet; gut microbiota; inflammation; post-cholecystectomy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

