MOF Template-Derived Carbon Shell-Embedded CoP Hierarchical Nanosheet as Bifunctional Catalyst for Overall Water Splitting
- PMID: 37686929
- PMCID: PMC10489850
- DOI: 10.3390/nano13172421
MOF Template-Derived Carbon Shell-Embedded CoP Hierarchical Nanosheet as Bifunctional Catalyst for Overall Water Splitting
Abstract
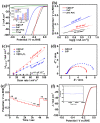
The design of earth-abundant and highly efficient bifunctional electrocatalysts for hydrogen evolution and oxygen evolution reactions (HER/OER) is crucial for hydrogen production through overall water splitting. Herein, we report a novel nanostructure consisting of vertically oriented CoP hierarchical nanosheet arrays with in situ-assembled carbon skeletons on a Ti foil electrode. The novel Zeolitic Imidazolate Framework-67 (ZIF-67) template-derived hierarchical nanosheet architecture effectively improved electrical conductivity, facilitated electrolyte transport, and increased the exposure of the active sites. The obtained bifunctional hybrid exhibited a low overpotential of 72 mV at 10 mA cm-2 and a small Tafel slope of 65 mV dec-1 for HER, and an improved overpotential of 329 mV and a Tafel slope of 107 mV dec-1 for OER. Furthermore, the assembled C@CoP||C@CoP electrolyzer showed excellent overall water splitting performance (1.63 V) at a current density of 10 mA cm-2 and superior durability. This work provides a structure engineering strategy for metal-organic framework (MOF) template-derived hybrids with outstanding electrocatalytic performance.
Keywords: hierarchical nanosheet; metal–organic framework; overall water splitting; transition metal phosphide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Bo X., Hocking R.K., Zhou S., Li Y., Chen X., Zhuang J., Du Y., Zhao C. Capturing the active sites of multimetallic (oxy)hydroxides for the oxygen evolution reaction. Energy Environ. Sci. 2020;13:4225–4237. doi: 10.1039/D0EE01609H. - DOI
-
- Vijayakumar E., Ramakrishnan S., Sathiskumar C., Yoo D.J., Balamurugan J., Noh H.S., Kwon D., Kim Y.H., Lee H. MOF-derived CoP-nitrogen-doped carbon@NiFeP nanoflakes as an efficient and durable electrocatalyst with multiple catalytically active sites for OER, HER, ORR and rechargeable zinc-air batteries. Chem. Eng. J. 2022;428:131115. doi: 10.1016/j.cej.2021.131115. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

