Mesoporous Dual-Semiconductor ZnS/CdS Nanocomposites as Efficient Visible Light Photocatalysts for Hydrogen Generation
- PMID: 37686934
- PMCID: PMC10490144
- DOI: 10.3390/nano13172426
Mesoporous Dual-Semiconductor ZnS/CdS Nanocomposites as Efficient Visible Light Photocatalysts for Hydrogen Generation
Abstract
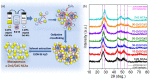
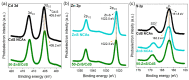
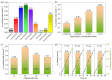
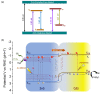
The development of functional catalysts for the photogeneration of hydrogen (H2) via water-splitting is crucial in the pursuit of sustainable energy solutions. To that end, metal-sulfide semiconductors, such as CdS and ZnS, can play a significant role in the process due to their interesting optoelectronic and catalytic properties. However, inefficient charge-carrier dissociation and poor photochemical stability remain significant limitations to photocatalytic efficiency. Herein, dual-semiconductor nanocomposites of ZnS/CdS nanocrystal assemblies (NCAs) are developed as efficient visible light photocatalysts for H2 generation. The resultant materials, synthesized via a polymer-templated self-polymerization method, comprise a unique combination of ~5-7 nm-sized metal-sulfide nanoparticles that are interlinked to form a 3D open-pore structure with large internal surface area (up to 285 m2 g-1) and uniform pores (circa 6-7 nm). By adjusting the ratio of constituent nanoparticles, the optimized ZnS/CdS catalyst with 50 wt.% ZnS content demonstrates a remarkable stability and visible light H2-evolution activity (~29 mmol g-1 h-1 mass activity) with an apparent quantum yield (AQY) of 60% at 420 nm. Photocatalytic evaluation experiments combined with electrochemical and spectroscopic studies suggest that the superior photocatalytic performance of these materials stems from the accessible 3D open-pore structure and the efficient defect-mediated charge transfer mechanism at the ZnS/CdS nanointerfaces. Overall, this work provides a new perspective for designing functional and stable photocatalytic materials for sustainable H2 production.
Keywords: ZnS/CdS heterojunctions; cadmium sulfide; mesoporous materials; metal chalcogenides; nanocomposites; nanoparticles; photocatalytic hydrogen production; water splitting; zinc sulfide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Höök M., Tang X. Depletion of Fossil Fuels and Anthropogenic Climate Change—A Review. Energy Policy. 2013;52:797–809. doi: 10.1016/j.enpol.2012.10.046. - DOI
-
- Mazloomi K., Gomes C. Hydrogen as an Energy Carrier: Prospects and Challenges. Renew. Sustain. Energy Rev. 2012;16:3024–3033. doi: 10.1016/j.rser.2012.02.028. - DOI
-
- Li J., Wu N. Semiconductor-Based Photocatalysts and Photoelectrochemical Cells for Solar Fuel Generation: A Review. Catal. Sci. Technol. 2015;5:1360–1384. doi: 10.1039/C4CY00974F. - DOI
LinkOut - more resources
Full Text Sources

