Microwave-Assisted Synthesis of Pt/SnO2 for the Catalytic Reduction of 4-Nitrophenol to 4-Aminophenol
- PMID: 37686989
- PMCID: PMC10489642
- DOI: 10.3390/nano13172481
Microwave-Assisted Synthesis of Pt/SnO2 for the Catalytic Reduction of 4-Nitrophenol to 4-Aminophenol
Abstract
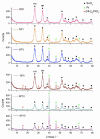
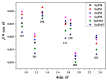
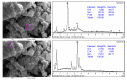
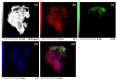
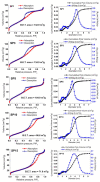
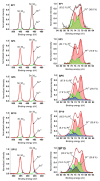
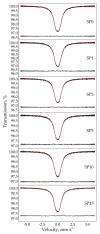
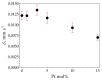
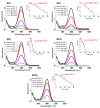
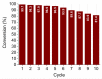
In this study, we present a new approach for the synthesis of Pt/SnO2 catalysts using microwave radiation. Pt(IV) and Sn(IV) inorganic precursors (H2PtCl6 and SnCl4) and ammonia were used, which allowed the controlled formation of platinum particles on the anisotropic SnO2 support. The synthesized Pt/SnO2 samples are mesoporous and exhibit a reversible physisorption isotherm of type IV. The XRD patterns confirmed the presence of platinum maxima in all Pt/SnO2 samples. The Williamson-Hall diagram showed SnO2 anisotropy with crystallite sizes of ~10 nm along the c-axis (< 00l >) and ~5 nm along the a-axis (< h00 >). SEM analysis revealed anisotropic, urchin-like SnO2 particles. XPS results indicated relatively low average oxidation states of platinum, close to Pt metal. 119Sn Mössbauer spectroscopy indicated electronic interactions between Pt and SnO2 particles. The synthesized samples were used for the catalytic reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) in the presence of excess NaBH4. The catalytic activity of the Pt/SnO2 samples for the reduction of 4-NP to 4-AP was optimized by varying the synthesis parameters and Pt loading. The optimal platinum loading for the reduction of 4-NP to 4-AP on the anisotropic SnO2 support is 5 mol% with an apparent rate constant k = 0.59 × 10-2 s-1. The Pt/SnO2 sample showed exceptional reusability and retained an efficiency of 81.4% after ten cycles.
Keywords: 119Sn Mössbauer; 4-nitrophenol; SnO2; XPS; catalyst; microwave synthesis; platinum.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Papandrew A.B., Chisholm C.R.I., Elgammal R.A., Özer M.M., Zecevic S.K. Advanced Electrodes for Solid Acid Fuel Cells by Platinum Deposition on CsH2PO4. Chem. Mater. 2011;23:1659–1667. doi: 10.1021/cm101147y. - DOI
-
- Liu D., Li X., Chen S., Yan H., Wang C., Wu C., Haleem Y.A., Duan S., Lu J., Ge B., et al. Atomically dispersed platinum supported on curved carbon supports for efficient electrocatalytic hydrogen evolution. Nat. Energy. 2019;4:512–518. doi: 10.1038/s41560-019-0402-6. - DOI
-
- Haneda M., Watanabe T., Kamiuchi N., Ozawa M. Effect of platinum dispersion on the catalytic activity of Pt/Al2O3 for the oxidation of carbon monoxide and propene. Appl. Catal. 2013;142:8–14. doi: 10.1016/j.apcatb.2013.04.055. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

