The Synthesis and Base-Induced Breakdown of Triaryl 1,4-Oxathiins-An Experimental and DFT Study
- PMID: 37687009
- PMCID: PMC10489040
- DOI: 10.3390/molecules28176180
The Synthesis and Base-Induced Breakdown of Triaryl 1,4-Oxathiins-An Experimental and DFT Study
Abstract
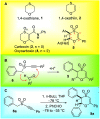
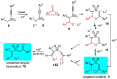
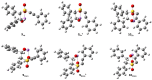
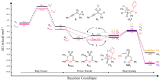
1,4-Oxathiins are valued for a breadth of bioactivities and are known commercial fungicides. This article explores a novel preparation of 2,3,6-trisubstituted 1,4-oxathiin-S,S-dioxides via the reaction of benzyl 1-alkynyl sulfones and aryl aldehydes under basic conditions. A total of 20 examples possessing exclusively a trans arrangement of the 2,3-diaryl substituents are exhibited; the products demonstrate a variation of functional groups on the aryl ring attached to the heterocyclic ring system. The preparation is hindered by the base sensitivity of the products, and a ring-opened by-product typically contaminates the reaction mixture. A DFT assessment of the overall system includes a lithium counterion and offers possible pathways for the incorporation of the aldehyde, the cyclization step and the requisite proton transfers. In addition, the DFT work reveals options for the ring opening chemistry. It appears the trans 2,3-diaryl selectivity is set during the cyclization stage of the reaction sequence. The practical work uncovers a new reaction pathway to create a family of novel 1,4-oxathiin-S,S-dioxides whereas the computational work offers an understanding of the structures and possible mechanisms involved.
Keywords: DFT; computational chemistry; cyclization; mechanism; oxathiin; sulfone; α-sulfonyl anion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Guillaumet G. Comprehensive Heterocyclic Chemistry II. Volume 6. Elsevier; Amsterdam, The Netherlands: 1996. 1,4-Dioxins, oxathiins, dithiins and their benzo derivatives; pp. 447–481, 1177–1307.
-
- Caputo R., Ferreri C., Guaragna A., Palumbo G., Pedatella S. New synthesis of carboxin and oxycarboxin pesticides: Application to the preparation of their new analogs substituted at the C-2 methyl group. J. Chem. Soc. Perkin Trans. 1. 1995:1971–1973. doi: 10.1039/p19950001971. - DOI
-
- Graham B.A., Puttock M.A., Felauer E.E., Neidermyer R.W. Substituted 2,3-dihydro-1,4-oxathiin as Plant Growth Regulators. DE2513202A1. 1975 October 23;
-
- Jaiswal A.K., Faisal M., Tailor S.P. Environment conscious control of Fusarium oxysporum F. sp. Lycopersici-induced tomato wilt using bio agents, phytochemicals and their combination in marked contrast to chemical. Pharma Innov. 2023;12:481–488. doi: 10.22271/tpi.2023.v12.i3e.18975. - DOI
LinkOut - more resources
Full Text Sources

