Synthesis of Aminobisphosphinates through a Cascade Reaction between Hypophosphorous Acid and Bis(trimethylsilyl)imidates Mediated by ZnI2
- PMID: 37687054
- PMCID: PMC10489009
- DOI: 10.3390/molecules28176226
Synthesis of Aminobisphosphinates through a Cascade Reaction between Hypophosphorous Acid and Bis(trimethylsilyl)imidates Mediated by ZnI2
Abstract
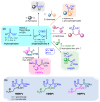
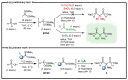
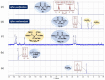
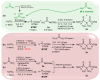
Among phosphorylated derivatives, phosphinates occupy a prominent place due to their ability to be bioisosteres of phosphates and carboxylates. These properties imply the necessity to develop efficient methodologies leading to phosphinate scaffolds. In recent years, our team has explored the nucleophilic potential of silylated phosphonite towards various electrophiles. In this paper, we propose to extend our study to other electrophiles. We describe here the implementation of a cascade reaction between (trimethylsilyl)imidates and hypophosphorous acid mediated by a Lewis acid allowing the synthesis of aminomethylenebisphosphinate derivatives. The present study focuses on methodological development including a careful NMR monitoring of the cascade reaction. The optimized conditions were successfully applied to various aliphatic and aromatic substituted (trimethylsilyl)imidates, leading to the corresponding AMBPi in moderate to good yields.
Keywords: Lewis acid; bisphosphinates; methodological development; phosphonite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Montchamp J.-L. Challenges and solutions in phosphinate chemistry. Pure Appl. Chem. 2019;91:113–120. doi: 10.1515/pac-2018-0922. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

