The Multifaceted Opportunities Provided by the Pheomelanin-Inspired 1,4-Benzothiazine Chromophore: A Still-Undervalued Issue
- PMID: 37687069
- PMCID: PMC10488698
- DOI: 10.3390/molecules28176237
The Multifaceted Opportunities Provided by the Pheomelanin-Inspired 1,4-Benzothiazine Chromophore: A Still-Undervalued Issue
Abstract
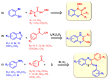
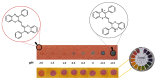
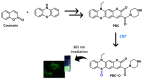
1,4-Benzothiazines are the main building blocks of the naturally occurring pheomelanin pigments, and their chromophoric properties have been strongly related to the well-known phototoxicity of these pigments, partly responsible for the high incidence of melanoma and other skin cancers in red-haired people. However, some peculiar features of the 1,4-benzothiazine chromophore could be functionally exploited in several sectors. Within this context, in this perspective, an overview of the very recently reported applications of the 1,4-benzothiazine chromophore in pH sensing, filter permeability control, smart packaging, electrochromic device fabrication, bioimaging, photocatalysis, and HPLC detection systems is provided, together with a brief presentation of recently developed synthetic approaches to the 1,4-benzothiazine scaffold, with the aim of emphasizing the still-undervalued multifunctional opportunities offered by this class of compounds.
Keywords: 1,4-benzothiazine; 2-aminothiophenol; acidichromism; bioimaging; electrochromism; pH sensing; pheomelanin; photocatalysis; smart packaging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





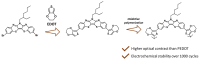


References
-
- Sharma P.K., Amin A., Kumar M. A review: Medicinally important nitrogen sulphur containing heterocycles. Open Med. Chem. J. 2020;14:49–64. doi: 10.2174/1874104502014010049. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

