Membrane-Targeting Perylenylethynylphenols Inactivate Medically Important Coronaviruses via the Singlet Oxygen Photogeneration Mechanism
- PMID: 37687107
- PMCID: PMC10488391
- DOI: 10.3390/molecules28176278
Membrane-Targeting Perylenylethynylphenols Inactivate Medically Important Coronaviruses via the Singlet Oxygen Photogeneration Mechanism
Abstract
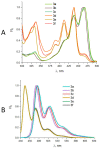
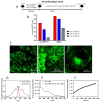
Perylenylethynyl derivatives have been recognized as broad-spectrum antivirals that target the lipid envelope of enveloped viruses. In this study, we present novel perylenylethynylphenols that exhibit nanomolar or submicromolar antiviral activity against Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) and feline infectious peritonitis virus (FIPV) in vitro. Perylenylethynylphenols incorporate into viral and cellular membranes and block the entry of the virus into the host cell. Furthermore, these compounds demonstrate an ability to generate singlet oxygen when exposed to visible light. The rate of singlet oxygen production is positively correlated with antiviral activity, confirming that the inhibition of fusion is primarily due to singlet-oxygen-induced damage to the viral envelope. The unique combination of a shape that affords affinity to the lipid bilayer and the capacity to generate singlet oxygen makes perylenylethynylphenols highly effective scaffolds against enveloped viruses. The anticoronaviral activity of perylenylethynylphenols is strictly light-dependent and disappears in the absence of daylight (under red light). Moreover, these compounds exhibit negligible cytotoxicity, highlighting their significant potential for further exploration of the precise antiviral mechanism and the broader scope and limitations of this compound class.
Keywords: SARS-CoV-2; antivirals; perylene; photosensitizers; singlet oxygen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

