Six New Phenolic Glycosides from the Seeds of Moringa oleifera Lam. and Their α-Glucosidase Inhibitory Activity
- PMID: 37687255
- PMCID: PMC10489651
- DOI: 10.3390/molecules28176426
Six New Phenolic Glycosides from the Seeds of Moringa oleifera Lam. and Their α-Glucosidase Inhibitory Activity
Abstract
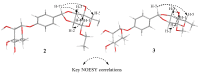
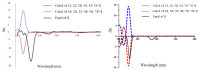
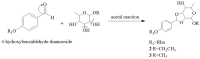
Plant-derived phytochemicals have recently drawn interest in the prevention and treatment of diabetes mellitus (DM). The seeds of Moringa oleifera Lam. are widely used in food and herbal medicine for their health-promoting properties against various diseases, including DM, but many of their effective constituents are still unknown. In this study, 6 new phenolic glycosides, moringaside B-G (1-6), together with 10 known phenolic glycosides (7-16) were isolated from M. oleifera seeds. The structures were elucidated by 1D and 2D NMR spectroscopy and high-resolution electrospray ionization mass spectrometry (HR-ESI-MS) data analysis. The absolute configurations of compounds 2 and 3 were determined by electronic circular dichroism (ECD) calculations. Compounds 2 and 3 especially are combined with a 1,3-dioxocyclopentane moiety at the rhamnose group, which are rarely reported in phenolic glycoside backbones. A biosynthetic pathway of 2 and 3 was assumed. Moreover, all the isolated compounds were evaluated for their inhibitory activities against α-glucosidase. Compounds 4 and 16 exhibited marked activities with IC50 values of 382.8 ± 1.42 and 301.4 ± 6.22 μM, and the acarbose was the positive control with an IC50 value of 324.1 ± 4.99 μM. Compound 16 revealed better activity than acarbose.
Keywords: chemical constituents; phenolic glycosides; seeds of Moringa oleifera Lam; structure identification; α-glucosidase inhibition activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Chiranjeev S., Youllee K., Dohee A., Sang J.C. Protein tyrosine phosphatases (PTPs) in diabetes: Causes and therapeutic opportunities. Arch. Pharm. Res. 2021;44:310–321. - PubMed
-
- Dehghan H., Salehi P., Amiri M.S. Bioassay-guided purification of α-amylase, α-glucosidase inhibitors and DPPH radical scavengers from roots of Rheum turkestanicum. Ind. Crops Prod. 2018;117:303–309. doi: 10.1016/j.indcrop.2018.02.086. - DOI
-
- Khoo C.M. Diabetes Mellitus Treatment. Int. Encycl. Public Health. 2017;2:288–293.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

