Effects of triclabendazole and its metabolite exposure on the heart-rate-corrected QT intervals: A randomized, placebo- and positive-controlled thorough QT study in healthy individuals
- PMID: 37688315
- PMCID: PMC10582667
- DOI: 10.1111/cts.13564
Effects of triclabendazole and its metabolite exposure on the heart-rate-corrected QT intervals: A randomized, placebo- and positive-controlled thorough QT study in healthy individuals
Abstract
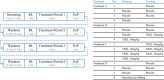
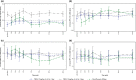
Triclabendazole is an effective and well-tolerated treatment for human fascioliasis. A placebo- and positive-controlled, four-sequence by four-period crossover study was conducted in 45 healthy participants to assess the effect of therapeutic (10 mg/kg twice daily [b.i.d.] for 1 day) and supratherapeutic (10 mg/kg b.i.d. for 3 days) oral doses of triclabendazole on corrected QT (QTc) interval prolongation. Moxifloxacin (400 mg, oral) was used as a positive control. The highest mean placebo-corrected change from baseline in QTcF (ΔΔQTcF) on day 4 with triclabendazole was 9.2 at therapeutic dose ms and 21.7 ms at supratherapeutic dose, at 4 h postdose. The upper limit of the two-sided 90% confidence interval exceeded 10 ms across all timepoints, except at predose timepoint on day 4 in the therapeutic group indicating that an effect of triclabendazole on cardiac repolarization could not be excluded. However, triclabendazole had no clinically significant effects on heart rate and cardiac conduction at the studied doses. In the moxifloxacin group, the mean ΔΔQTcF peak value was 13.7 ms at 3 h on day 4. The assay sensitivity was confirmed. Maximum plasma concentration of triclabendazole, sulfoxide metabolite, and sulfone metabolite occurred at ~3-, 4-, and 6-h postdose, respectively. No deaths, serious adverse events, study discontinuations due to treatment-emergent adverse events, or clinically relevant abnormalities in laboratory evaluations and vital sign values were observed. This study showed that triclabendazole had no clinically relevant effects on heart rate and cardiac conduction; however, an effect on cardiac repolarization (ΔΔQTcF >10 ms) could not be excluded.
© 2023 NOVARTIS. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
All authors are employees of Novartis.
Figures

Similar articles
-
Lack of effect of perampanel on QT interval duration: Results from a thorough QT analysis and pooled partial seizure Phase III clinical trials.Epilepsy Res. 2015 Aug;114:122-30. doi: 10.1016/j.eplepsyres.2015.04.010. Epub 2015 May 1. Epilepsy Res. 2015. PMID: 26088895 Clinical Trial.
-
Effects of Therapeutic and Supratherapeutic Doses of Siponimod (BAF312) on Cardiac Repolarization in Healthy Subjects.Clin Ther. 2015 Nov 1;37(11):2489-2505.e2. doi: 10.1016/j.clinthera.2015.09.006. Epub 2015 Oct 27. Clin Ther. 2015. PMID: 26519230 Clinical Trial.
-
Effect of Retosiban on Cardiac Repolarization in a Randomized, Placebo- and Positive-controlled, Crossover Thorough QT/QTc Study in Healthy Men and Women.Clin Ther. 2015 Jul 1;37(7):1541-54. doi: 10.1016/j.clinthera.2015.05.007. Epub 2015 Jun 29. Clin Ther. 2015. PMID: 26138866 Clinical Trial.
-
A Thorough QT Study of the Combination Glecaprevir + Pibrentasvir on Cardiac Repolarization in Healthy Subjects.Clin Ther. 2020 Jul;42(7):1317-1329. doi: 10.1016/j.clinthera.2020.05.009. Epub 2020 Jul 1. Clin Ther. 2020. PMID: 32622784 Clinical Trial.
-
Triclabendazole.2021 Sep 1. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. 2021 Sep 1. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. PMID: 34591438 Free Books & Documents. Review.
References
-
- Keiser J, Engels D, Buscher G, Utzinger J. Triclabendazole for the treatment of fascioliasis and paragonimiasis. Expert Opin Investig Drugs. 2005;14:1513‐1526. - PubMed
-
- CDC–Fasciola–Resources for Health Professionals . https://www.cdc.gov/parasites/fasciola/health_professionals/index.html#tx. Accessed October 6, 2021.
-
- US FDA drug trials snapshots: EGATEN . https://www.fda.gov/drugs/drug‐trials‐snapshots‐egaten. Accessed October 6, 2021.
-
- Fascioliasis diagnosis, treatment and control strategy . https://www.who.int/foodborne_trematode_infections/fascioliasis/fascioli.... Accessed October 6, 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

