Systematic evidence review and meta-analysis of outcomes associated with cancer genetic counseling
- PMID: 37688462
- PMCID: PMC11981685
- DOI: 10.1016/j.gim.2023.100980
Systematic evidence review and meta-analysis of outcomes associated with cancer genetic counseling
Abstract
Purpose: Genetic counseling (GC) is standard of care in genetic cancer risk assessment (GCRA). A rigorous assessment of the data reported from published studies is crucial to ensure the evidence-based implementation of GC.
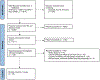
Methods: We conducted a systematic review and meta-analysis of 17 patient-reported and health-services-related outcomes associated with pre- and post-test GC in GCRA in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology.
Results: Twenty-five of 5393 screened articles met inclusion criteria. No articles reporting post-test GC outcomes met inclusion criteria. For patient-reported outcomes, pre-test GC significantly decreased worry, increased knowledge, and decreased perceived risk but did not significantly affect patient anxiety, depression, decisional conflict, satisfaction, or intent to pursue genetic testing. For health-services outcomes, pre-test GC increased correct genetic test ordering, reduced inappropriate services, increased spousal support for genetic testing, and expedited care delivery but did not consistently improve cancer prevention behaviors nor lead to accurate risk assessment. The GRADE certainty in the evidence was very low or low. No included studies elucidated GC effect on mortality, cascade testing, cost-effectiveness, care coordination, shared decision making, or patient time burden.
Conclusion: The true impact of GC on relevant outcomes is not known low quality or absent evidence. Although a meta-analysis found that pre-test GC had beneficial effects on knowledge, worry, and risk perception, the certainty of this evidence was low according to GRADE methodology. Further studies are needed to support the evidence-based application of GC in GCRA.
Keywords: Cancer genetics; GRADE; Genetic counseling; Health services; Outcomes; PRISMA.
Copyright © 2023 American College of Medical Genetics and Genomics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Julie O. Culver, Nicole L. Bertsch, Raluca N. Kurz, Smita Rao, Shannon Stasi, Chris D. Stave, and Ravi N. Sharaf have no conflicts of interest to declare. At the time of the systematic review, Linda L. Cheng was an employee of Quest Diagnostics and received salary from Quest Diagnostics. Mary Pritzlaff is an employee and receives full time salary from Ambry Genetics and is an intellectual property owner and receives royalties for CancerGene Connect.
Figures
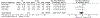
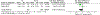
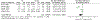
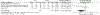
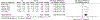
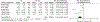
Similar articles
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Physical activity for treatment of irritable bowel syndrome.Cochrane Database Syst Rev. 2022 Jun 29;6(6):CD011497. doi: 10.1002/14651858.CD011497.pub2. Cochrane Database Syst Rev. 2022. PMID: 35766861 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Early palliative care for adults with advanced cancer.Cochrane Database Syst Rev. 2017 Jun 12;6(6):CD011129. doi: 10.1002/14651858.CD011129.pub2. Cochrane Database Syst Rev. 2017. PMID: 28603881 Free PMC article.
Cited by
-
Identification of Patients at Elevated Cancer Risk through a Community-Based Genetic Testing Program.Ann Surg Oncol. 2025 Aug 6. doi: 10.1245/s10434-025-17820-w. Online ahead of print. Ann Surg Oncol. 2025. PMID: 40770526
-
Call to action for genetic counseling research in hereditary cancer: Considerations from the evidence-based guidelines development process.J Genet Couns. 2025 Jun;34(3):e70026. doi: 10.1002/jgc4.70026. J Genet Couns. 2025. PMID: 40305378 Free PMC article.
-
The efficacy of genetic counseling for familial colorectal cancer: A meta-analysis.J Genet Couns. 2025 Jun;34(3):e70046. doi: 10.1002/jgc4.70046. J Genet Couns. 2025. PMID: 40459217 Free PMC article.
References
-
- NCCN. Genetic Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic v.3.2023. Principles of Cancer Risk Assessment and Counseling: National Comprehensive Cancer Network; 2023.
-
- Centers NAPFB. National Accreditation Program for Breast Centers Standards Manual. Genetic Evaluation and Management. Chicago, IL: American College of Surgeons; 2018:3.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

