Both stronger and weaker cerebro-cerebellar functional connectivity patterns during processing of spoken sentences in autism spectrum disorder
- PMID: 37688547
- PMCID: PMC10619366
- DOI: 10.1002/hbm.26478
Both stronger and weaker cerebro-cerebellar functional connectivity patterns during processing of spoken sentences in autism spectrum disorder
Abstract
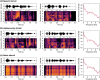
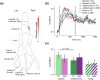
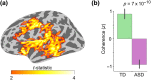
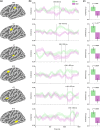
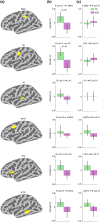
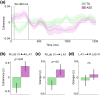
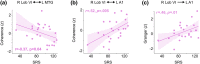
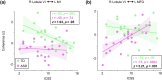
Cerebellar differences have long been documented in autism spectrum disorder (ASD), yet the extent to which such differences might impact language processing in ASD remains unknown. To investigate this, we recorded brain activity with magnetoencephalography (MEG) while ASD and age-matched typically developing (TD) children passively processed spoken meaningful English and meaningless Jabberwocky sentences. Using a novel source localization approach that allows higher resolution MEG source localization of cerebellar activity, we found that, unlike TD children, ASD children showed no difference between evoked responses to meaningful versus meaningless sentences in right cerebellar lobule VI. ASD children also had atypically weak functional connectivity in the meaningful versus meaningless speech condition between right cerebellar lobule VI and several left-hemisphere sensorimotor and language regions in later time windows. In contrast, ASD children had atypically strong functional connectivity for in the meaningful versus meaningless speech condition between right cerebellar lobule VI and primary auditory cortical areas in an earlier time window. The atypical functional connectivity patterns in ASD correlated with ASD severity and the ability to inhibit involuntary attention. These findings align with a model where cerebro-cerebellar speech processing mechanisms in ASD are impacted by aberrant stimulus-driven attention, which could result from atypical temporal information and predictions of auditory sensory events by right cerebellar lobule VI.
Keywords: MEG; autism; cerebellum; functional connectivity; speech.
© 2023 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Alho, J. , Bharadwaj, H. , Khan, S. , Mamashli, F. , Perrachione, T. K. , Losh, A. , McGuiggan, N. M. , Joseph, R. M. , Hämäläinen, M. S. , & Kenet, T. (2021). Altered maturation and atypical cortical processing of spoken sentences in autism spectrum disorder. Progress in Neurobiology, 203, 102077. - PMC - PubMed
-
- Alho, J. , Khan, S. , Mamashli, F. , Perrachione, T. K. , Losh, A. , McGuiggan, N. M. , Graham, S. , Nayal, Z. , Joseph, R. M. , Hämäläinen, M. S. , Bharadwaj, H. , & Kenet, T. (2023). Atypical cortical processing of bottom‐up speech binding cues in children with autism spectrum disorders. NeuroImage Clinical, 37, 103336. - PMC - PubMed
-
- Arnold Anteraper, S. , Guell, X. , D'Mello, A. , Joshi, N. , Whitfield‐Gabrieli, S. , & Joshi, G. (2019). Disrupted cerebrocerebellar intrinsic functional connectivity in young adults with high‐functioning autism spectrum disorder: A data‐driven, whole‐brain, high‐temporal resolution functional magnetic resonance imaging study. Brain Connectivity, 9, 48–59. - PubMed

