Irisin reduces amyloid-β by inducing the release of neprilysin from astrocytes following downregulation of ERK-STAT3 signaling
- PMID: 37689059
- PMCID: PMC10840702
- DOI: 10.1016/j.neuron.2023.08.012
Irisin reduces amyloid-β by inducing the release of neprilysin from astrocytes following downregulation of ERK-STAT3 signaling
Abstract
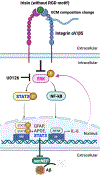
A pathological hallmark of Alzheimer's disease (AD) is the deposition of amyloid-β (Aβ) protein in the brain. Physical exercise has been shown to reduce Aβ burden in various AD mouse models, but the underlying mechanisms have not been elucidated. Irisin, an exercise-induced hormone, is the secreted form of fibronectin type-III-domain-containing 5 (FNDC5). Here, using a three-dimensional (3D) cell culture model of AD, we show that irisin significantly reduces Aβ pathology by increasing astrocytic release of the Aβ-degrading enzyme neprilysin (NEP). This is mediated by downregulation of ERK-STAT3 signaling. Finally, we show that integrin αV/β5 acts as the irisin receptor on astrocytes required for irisin-induced release of astrocytic NEP, leading to clearance of Aβ. Our findings reveal for the first time a cellular and molecular mechanism by which exercise-induced irisin attenuates Aβ pathology, suggesting a new target pathway for therapies aimed at the prevention and treatment of AD.
Keywords: Alzheimer's disease; Aβ; ERK; STAT3; amyloid-β; astrocytes; integrin αV/β5; irisin; neprilysin.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare the following competing interests: B.M.S. and C.D.W. hold a patent related to irisin (WO2015051007A1). B.M.S. and C.D.W. are academic co-founders and consultants for Aevum Therapeutics. C.D.W. has a financial interest in Aevum Therapeutics, a company developing drugs that harness the protective molecular mechanisms of exercise to treat neurodegenerative and neuromuscular disorders. C.D.W.’s interests were reviewed and are managed by MGH and Mass General Brigham in accordance with their conflict-of-interest policies.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

