The Role of Endoplasmic Reticulum in Lipotoxicity during Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Pathogenesis
- PMID: 37689385
- PMCID: PMC10699131
- DOI: 10.1016/j.ajpath.2023.08.007
The Role of Endoplasmic Reticulum in Lipotoxicity during Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Pathogenesis
Abstract
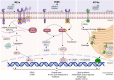
Perturbations in lipid and protein homeostasis induce endoplasmic reticulum (ER) stress in metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as nonalcoholic fatty liver disease. Lipotoxic and proteotoxic stress can activate the unfolded protein response (UPR) transducers: inositol requiring enzyme1α, PKR-like ER kinase, and activating transcription factor 6α. Collectively, these pathways induce expression of genes that encode functions to resolve the protein folding defect and ER stress by increasing the protein folding capacity of the ER and degradation of misfolded proteins. The ER is also intimately connected with lipid metabolism, including de novo ceramide synthesis, phospholipid and cholesterol synthesis, and lipid droplet formation. Following their activation, the UPR transducers also regulate lipogenic pathways in the liver. With persistent ER stress, cellular adaptation fails, resulting in hepatocyte apoptosis, a pathological marker of liver disease. In addition to the ER-nucleus signaling activated by the UPR, the ER can interact with other organelles via membrane contact sites. Modulating intracellular communication between ER and endosomes, lipid droplets, and mitochondria to restore ER homeostasis could have therapeutic efficacy in ameliorating liver disease. Recent studies have also demonstrated that cells can convey ER stress by the release of extracellular vesicles. This review discusses lipotoxic ER stress and the central role of the ER in communicating ER stress to other intracellular organelles in MASLD pathogenesis.
Copyright © 2023 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure Statement None declared.
Figures


References
-
- Riazi K., Azhari H., Charette J.H., Underwood F.E., King J.A., Afshar E.E., Swain M.G., Congly S.E., Kaplan G.G., Shaheen A.A. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851–861. - PubMed
-
- Malhotra J.D., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

