Natural diversity of lactococci in γ-aminobutyric acid (GABA) production and genetic and phenotypic determinants
- PMID: 37689693
- PMCID: PMC10492284
- DOI: 10.1186/s12934-023-02181-4
Natural diversity of lactococci in γ-aminobutyric acid (GABA) production and genetic and phenotypic determinants
Abstract
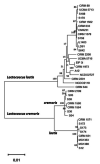
Background: γ-aminobutyric acid (GABA) is a bioactive compound produced by lactic acid bacteria (LAB). The diversity of GABA production in the Lactococcus genus is poorly understood. Genotypic and phenotypic approaches were therefore combined in this study to shed light on this diversity. A comparative genomic study was performed on the GAD-system genes (gadR, gadC and gadB) involved in GABA production in 36 lactococci including L. lactis and L. cremoris species. In addition, 132 Lactococcus strains were screened for GABA production in culture medium supplemented with 34 mM L-glutamic acid with or without NaCl (0.3 M).
Results: Comparative analysis of the nucleotide sequence alignments revealed the same genetic organization of the GAD system in all strains except one, which has an insertion sequence element (IS981) into the PgadCB promoter. This analysis also highlighted several deletions including a 3-bp deletion specific to the cremoris species located in the PgadR promoter, and a second 39-bp deletion specific to L. cremoris strains with a cremoris phenotype. Phenotypic analysis revealed that GABA production varied widely, but it was higher in L. lactis species than in L. cremoris, with an exceptional GABA production of up to 14 and 24 mM in two L. lactis strains. Moreover, adding chloride increased GABA production in some L. cremoris and L. lactis strains by a factor of up to 16 and GAD activity correlated well with GABA production.
Conclusions: This genomic analysis unambiguously characterized the cremoris phenotype of L. cremoris species and modified GadB and GadR proteins explain why the corresponding strains do not produce GABA. Finally, we found that glutamate decarboxylase activity revealing GadB protein amount, varied widely between the strains and correlated well with GABA production both with and without chloride. As this protein level is associated to gene expression, the regulation of GAD gene expression was identified as a major contributor to this diversity.
Keywords: GAD system; Lactococcus cremoris; Lactococcus lactis; γ-aminobutyric acid.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
The authors declare no competing interests.
Figures



References
-
- Kandler O. Carbohydrate metabolism in lactic acid bacteria. Antonie Van Leeuwenhoek. 1983;49:209–22. - PubMed
-
- Ruas-Madiedo P, de los Reyes-Gavilán CG. Invited review: methods for the screening, isolation, and characterization of Exopolysaccharides produced by lactic acid Bacteria. J Dairy Sci. 2005;88:843–56. - PubMed
-
- López-Cuellar Ma del R. Rodríguez-Hernández A-I, Chavarría-Hernández N. LAB bacteriocin applications in the last decade. Biotechnol Biotechnol Equipment. 2016;30:1039–50.
-
- Chen C, Zhao S, Hao G, Yu H, Tian H, Zhao G. Role of lactic acid bacteria on the yogurt flavour: a review. Int J Food Properties. 2017;20:316–30.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

