Asparagine endopeptidase protects podocytes in adriamycin-induced nephropathy by regulating actin dynamics through cleaving transgelin
- PMID: 37689970
- PMCID: PMC10638058
- DOI: 10.1016/j.ymthe.2023.09.003
Asparagine endopeptidase protects podocytes in adriamycin-induced nephropathy by regulating actin dynamics through cleaving transgelin
Abstract
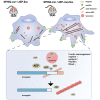
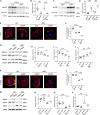
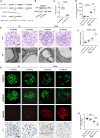
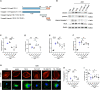
Focal segmental glomerulosclerosis (FSGS) is the most common glomerular disorder causing end-stage renal diseases worldwide. Central to the pathogenesis of FSGS is podocyte dysfunction, which is induced by diverse insults. However, the mechanism governing podocyte injury and repair remains largely unexplored. Asparagine endopeptidase (AEP), a lysosomal protease, regulates substrates by residue-specific cleavage or degradation. We identified the increased AEP expression in the primary proteinuria model which was induced by adriamycin (ADR) to mimic human FSGS. In vivo, global AEP knockout mice manifested increased injury-susceptibility of podocytes in ADR-induced nephropathy (ADRN). Podocyte-specific AEP knockout mice exhibited much more severe glomerular lesions and podocyte injury after ADR injection. In contrast, podocyte-specific augmentation of AEP in mice protected against ADRN. In vitro, knockdown and overexpression of AEP in human podocytes revealed the cytoprotection of AEP as a cytoskeleton regulator. Furthermore, transgelin, an actin-binding protein regulating actin dynamics, was cleaved by AEP, and, as a result, removed its actin-binding regulatory domain. The truncated transgelin regulated podocyte actin dynamics and repressed podocyte hypermotility, compared to the native full-length transgelin. Together, our data reveal a link between lysosomal protease AEP and podocyte cytoskeletal homeostasis, which suggests a potential therapeutic role for AEP in proteinuria disease.
Keywords: asparagine endopeptidase; cytoskeletal dynamics; focal segmental glomerulosclerosis; lysosomal protease; podocyte injury; transgelin.
Copyright © 2023 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests All authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

