Bioactive Suture with Added Innate Defense Functionality for the Reduction of Bacterial Infection and Inflammation
- PMID: 37689972
- PMCID: PMC11468473
- DOI: 10.1002/adhm.202300987
Bioactive Suture with Added Innate Defense Functionality for the Reduction of Bacterial Infection and Inflammation
Abstract
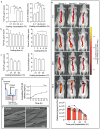
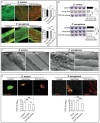
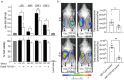
Surgical site infections (SSI) are a clinical and economic burden. Suture-associated SSI may develop when bacteria colonize the suture surface and form biofilms that are resistant to antibiotics. Thrombin-derived C-terminal peptide (TCP)-25 is a host defense peptide with a unique dual mode of action that can target both bacteria and the excessive inflammation induced by bacterial products. The peptide demonstrates therapeutic potential in preclinical in vivo wound infection models. In this study, the authors set out to explore whether TCP-25 can provide a new bioactive innate immune feature to hydrophilic polyglactin sutures (Vicryl). Using a combination of biochemical, biophysical, antibacterial, biofilm, and anti-inflammatory assays in vitro, in silico molecular modeling studies, along with experimental infection and inflammation models in mice, a proof-of-concept that TCP-25 can provide Vicryl sutures with a previously undisclosed host defense capacity, that enables targeting of bacteria, biofilms, and the accompanying inflammatory response, is shown.
Keywords: TCP-25; host defense peptides; polyglactin; surgical site infections; suture.
© 2023 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
A.S. is one of the founders of in2cure AB, a company developing peptides for therapeutic applications. A patent application has been filed on materials with anti‐inflammatory and/or anti‐microbial properties.
Figures






References
-
- a) Weiser T. G., Haynes A. B., Molina G., Lipsitz S. R., Esquivel M. M., Uribe‐Leitz T., Fu R., Azad T., Chao T. E., Berry W. R., Gawande A. A., Bull. W. H. O. 2016, 94, 201; - PMC - PubMed
- b) Dobson G. P., Int. J. Surg. 2020, 81, 47; - PMC - PubMed
- c) Weiser T. G., Haynes A. B., Molina G., Lipsitz S. R., Esquivel M. M., Uribe‐Leitz T., Fu R., Azad T., Chao T. E., Berry W. R., Gawande A. A., Lancet 2015, 385, S11. - PubMed
-
- Gillespie B. M., Harbeck E., Rattray M., Liang R., Walker R., Latimer S., Thalib L., Andersson A. E., Griffin B., Ware R., Chaboyer W., Int. J. Surg. 2021, 95, 106136. - PubMed
-
- a) Owens C. D., Stoessel K., J. Hosp. Infect. 2008, 70, 3; - PubMed
- b) Surgery N. G. R. H. U. o. G., Lancet 2021, 398, 1687; - PubMed
- c) Allegranzi B., Nejad S. B., Combescure C., Graafmans W., Attar H., Donaldson L., Pittet D., Lancet 2011, 377, 228; - PubMed
- d) Kirkland K. B., Briggs J. P., Trivette S. L., Wilkinson W. E., Sexton D. J., Infect. Control Hosp. Epidemiol. 1999, 20, 725. - PubMed
-
- Allegranzi B., Bischoff P., De Jonge S., Kubilay N. Z., Zayed B., Gomes S. M., Abbas M., Atema J. J., Gans S., Van Rijen M., Boermeester M. A., Egger M., Kluytmans J., Pittet D., Solomkin J. S., Lancet Infect. Dis. 2016, 16, e276. - PubMed
-
- Surgery N. G. R. H. U. o. G., Lancet 2022, 400, 1767. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

