Cortex-restricted deletion of Foxp1 impairs barrel formation and induces aberrant tactile responses in a mouse model of autism
- PMID: 37691105
- PMCID: PMC10494400
- DOI: 10.1186/s13229-023-00567-0
Cortex-restricted deletion of Foxp1 impairs barrel formation and induces aberrant tactile responses in a mouse model of autism
Abstract
Background: Many children and young people with autism spectrum disorder (ASD) display touch defensiveness or avoidance (hypersensitivity), or engage in sensory seeking by touching people or objects (hyposensitivity). Abnormal sensory responses have also been noticed in mice lacking ASD-associated genes. Tactile sensory information is normally processed by the somatosensory system that travels along the thalamus to the primary somatosensory cortex. The neurobiology behind tactile sensory abnormalities, however, is not fully understood.
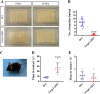
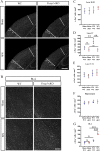
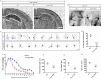
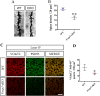
Methods: We employed cortex-specific Foxp1 knockout (Foxp1-cKO) mice as a model of autism in this study. Tactile sensory deficits were measured by the adhesive removal test. The mice's behavior and neural activity were further evaluated by the whisker nuisance test and c-Fos immunofluorescence, respectively. We also studied the dendritic spines and barrel formation in the primary somatosensory cortex by Golgi staining and immunofluorescence.
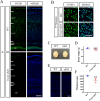
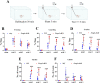
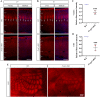
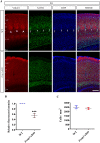
Results: Foxp1-cKO mice had a deferred response to the tactile environment. However, the mice exhibited avoidance behavior and hyper-reaction following repeated whisker stimulation, similar to a fight-or-flight response. In contrast to the wild-type, c-Fos was activated in the basolateral amygdala but not in layer IV of the primary somatosensory cortex of the cKO mice. Moreover, Foxp1 deficiency in cortical neurons altered the dendrite development, reduced the number of dendritic spines, and disrupted barrel formation in the somatosensory cortex, suggesting impaired somatosensory processing may underlie the aberrant tactile responses.
Limitations: It is still unclear how the defective thalamocortical connection gives rise to the hyper-reactive response. Future experiments with electrophysiological recording are needed to analyze the role of thalamo-cortical-amygdala circuits in the disinhibiting amygdala and enhanced fearful responses in the mouse model of autism.
Conclusions: Foxp1-cKO mice have tactile sensory deficits while exhibit hyper-reactivity, which may represent fearful and emotional responses controlled by the amygdala. This study presents anatomical evidence for reduced thalamocortical connectivity in a genetic mouse model of ASD and demonstrates that the cerebral cortex can be the origin of atypical sensory behaviors.
Keywords: Autism; Barrel cortex; Spines; Tactile; Thalamocortical; c-Fos.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors have no competing interests to declare.
Figures
References
-
- Ozonoff S. Editorial perspective: autism spectrum disorders in DSM-5–an historical perspective and the need for change. J Child Psychol Psychiatry. 2012;53:1092–1094. - PubMed
-
- Robertson CE, Baron-Cohen S. Sensory perception in autism. Nat Rev Neurosci. 2017;18:671–684. - PubMed
-
- Dunn W. The sensations of everyday life: empirical, theoretical, and pragmatic considerations. Am J Occup Ther. 2001;55:608–620. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

