Neuroprotective Effect of Tauroursodeoxycholic Acid (TUDCA) on In Vitro and In Vivo Models of Retinal Disorders: A Systematic Review
- PMID: 37691227
- PMCID: PMC11092919
- DOI: 10.2174/1570159X21666230907152207
Neuroprotective Effect of Tauroursodeoxycholic Acid (TUDCA) on In Vitro and In Vivo Models of Retinal Disorders: A Systematic Review
Abstract
Background: Tauroursodeoxycholic acid (TUDCA) is a naturally produced hydrophilic bile acid that has been used for centuries in Chinese medicine. Numerous recent in vitro and in vivo studies have shown that TUDCA has neuroprotective action in various models of retinal disorders.
Objective: To systematically review the scientific literature and provide a comprehensive summary on the neuroprotective action and the mechanisms involved in the cytoprotective effects of TUDCA.
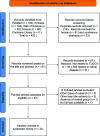
Methods: A systematic review was conducted in accordance with the PRISMA (The Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. Systematic literature search of United States National Library of Medicine (PubMed), Web of Science, Embase, Scopus and Cochrane Library was performed, which covered all original articles published up to July 2022. The terms, "TUDCA" in combination with "retina", "retinal protection", "neuroprotection" were searched. Possible biases were identified with the adopted SYRCLE's tool.
Results: Of the 423 initially gathered studies, 24 articles met inclusion/exclusion criteria for full-text review. Six of them were in vitro experiments, 17 studies reported in vivo data and one study described both in vitro and in vivo data. The results revealed the effect of TUDCA on different retinal diseases, such as retinitis pigmentosa (RP), diabetic retinopathy (DR), retinal degeneration (RD), retinal ganglion cell (RGC) injury, Leber's hereditary optic neuropathy (LHON), choroidal neovascularization (CNV), and retinal detachment (RDT). The quality scores of the in vivo studies were ranged from 5 to 7 points (total 10 points), according to SYRCLE's risk of bias tool. Both in vitro and in vivo data suggested that TUDCA could effectively delay degeneration and apoptosis of retinal neurons, preserve retinal structure and function, and its mechanism of actions might be related with inhibiting apoptosis, decreasing inflammation, attenuating oxidative stress, suppressing endoplasmic reticulum (ER) stress, and reducing angiogenesis.
Conclusion: This systematic review demonstrated that TUDCA has neuroprotective effect on in vivo and in vitro models of retinal disorders, reinforcing the currently available evidence that TUDCA could be a promising therapeutic agent in retinal diseases treatment. However, well designed clinical trials are necessary to appraise the efficacy of TUDCA in clinical setting.
Keywords: TUDCA; Tauroursodeoxycholic acid; diabetic retinopathy; neuroprotection; retinal degeneration; retinal disorder; retinitis pigmentosa; traditional Chinese medicine..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
The authors certify that they have no affiliation with or financial involvement in any organization with a direct financial interest in the subject matter or materials discussed in the manuscript.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

