Efficacy and safety of four-year ofatumumab treatment in relapsing multiple sclerosis: The ALITHIOS open-label extension
- PMID: 37691530
- PMCID: PMC10580679
- DOI: 10.1177/13524585231195346
Efficacy and safety of four-year ofatumumab treatment in relapsing multiple sclerosis: The ALITHIOS open-label extension
Abstract
Background: Ofatumumab has demonstrated superior efficacy and favorable safety for up to 2.5 years versus teriflunomide in relapsing multiple sclerosis (RMS).
Objective: Further characterize efficacy and safety of ofatumumab in RMS.
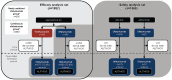
Methods: Efficacy set: patients randomized to ofatumumab/teriflunomide in ASCLEPIOS I/II (core). Safety set: patients who received ⩾ 1 dose of ofatumumab in ASCLEPIOS I/II, APLIOS, APOLITOS (all core), or ALITHIOS (umbrella open-label extension). Patients received continuous ofatumumab or were newly switched from teriflunomide. Data cut-off: 25 September 2021.
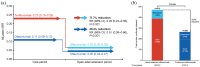
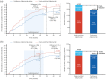
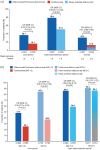
Results: In the efficacy set (n = 1882), the continuous ofatumumab group had a low annualized relapse rate (ARR 0.05 (95% confidence interval: 0.04-0.07)), low numbers of gadolinium-enhancing (Gd+) T1 lesions (0.01 lesions/scan) and fewer new/enlarging T2 lesions (annualized rate 0.08). Overall, 78.8% met three-parameter "no evidence of disease activity" criteria through 4 years. Switching from teriflunomide led to reduced ARR, risk of confirmed disability worsening (CDW), new/enlarging T2 lesions, Gd+ T1 lesions, and serum neurofilament light chain. In the continuous and newly switched ofatumumab groups, cumulative 3- and 6-month CDW rates remained low. In the safety set (n = 1969), the most frequently reported adverse events were infections and infestations (58.35%). No new safety signals were identified.
Conclusion: Ofatumumab has a favorable longer-term benefit-risk profile in RMS.
Trial registry: ALITHIOS (NCT03650114): https://clinicaltrials.gov/ct2/show/NCT03650114.
Keywords: Relapsing-remitting multiple sclerosis; follow-up; monoclonal antibodies; ofatumumab; treatment.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All support for the present manuscript (e.g. funding, provision of study materials, medical writing, article processing charges) and the studies were sponsored and funded by Novartis Pharma AG, Basel, Switzerland. Novartis Pharma AG supported the development of this manuscript, provided data analyses according to the direction of the authors, and provided funding for medical writing support. Open access fee was paid by Novartis. Medical writing support for the development of this publication, under the direction of the authors, was provided by James Currie (BSc, Hons; PhD.), Laura Crocker (BMedSci, Hons), and Philippa Lloyd (BSc, Hons) of Ashfield MedComms, an Inizio company, and was funded by Novartis Pharma AG. Stephen L Hauser currently serves on the scientific advisory board of ACCURE, Alector, Annexon, board of directors of Neurona, and has previously consulted for BD, Moderna, and NGM Bio. Dr Hauser also has received travel reimbursement and writing support from F. Hoffmann-La Roche and Novartis AG for anti-CD20-therapy-related meetings and presentations. Grants: NIH/NINDS (R35NS111644) and Valhalla Foundation; within the past 36 months, no longer active: National Multiple Sclerosis Society (RR 2005-A-13). Ronald Zielman is a full-time employee of Novartis. Ayan Das Gupta is a paid and permanent employee of Novartis. Jing Xi is an employee of Novartis. Dee Stoneman is a full-time employee of Novartis. Goeril Karlsson is a full-time salaried employee of Novartis. Derrick Robertson declares grants or contracts from Anokion, Atara Biotherapeutics, Biogen, GW Pharmaceuticals, Novartis, PRIME CME, TG Therapeutics, CorEvitas, EMD Serono, Genentech, Janssen, PCORI and Sanofi; consulting fees from Biogen, Genentech, EMD Serono, Janssen, Bristol Myers Squibb, Horizon, Novartis, Sanofi, TG Therapeutics, Alexion, Greenwich Biosciences and Mallinckrodt; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Biogen, EMD Serono, Genentech, TG Therapeutics, Bristol Myers Squibb, Janssen, PRIME CME, Sanofi, Alexion and Horizon. Jeffrey A Cohen declares consulting fees from Biogen, Convelo, EMD Serono, Gaossamer Gio, Mylan and PSA; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid for ACTRIMS; other financial or non-financial interests for Sage—serving as an Editor of
Figures

Similar articles
-
Five-Year Safety and Efficacy Outcomes with Ofatumumab in Patients with Relapsing Multiple Sclerosis.Neurol Ther. 2025 Jul 13. doi: 10.1007/s40120-025-00784-0. Online ahead of print. Neurol Ther. 2025. PMID: 40652442
-
Ofatumumab versus Teriflunomide in Multiple Sclerosis.N Engl J Med. 2020 Aug 6;383(6):546-557. doi: 10.1056/NEJMoa1917246. N Engl J Med. 2020. PMID: 32757523 Clinical Trial.
-
Efficacy and safety of ofatumumab in recently diagnosed, treatment-naive patients with multiple sclerosis: Results from ASCLEPIOS I and II.Mult Scler. 2022 Sep;28(10):1562-1575. doi: 10.1177/13524585221078825. Epub 2022 Mar 10. Mult Scler. 2022. PMID: 35266417 Free PMC article. Clinical Trial.
-
The Development of Ofatumumab, a Fully Human Anti-CD20 Monoclonal Antibody for Practical Use in Relapsing Multiple Sclerosis Treatment.Neurol Ther. 2023 Oct;12(5):1491-1515. doi: 10.1007/s40120-023-00518-0. Epub 2023 Jul 14. Neurol Ther. 2023. PMID: 37450172 Free PMC article. Review.
-
Efficacy, safety and patient reported outcomes in patients with active relapsing multiple sclerosis treated with ocrelizumab: Final results from the PRO-MSACTIVE study.Mult Scler Relat Disord. 2022 Dec;68:104109. doi: 10.1016/j.msard.2022.104109. Epub 2022 Aug 13. Mult Scler Relat Disord. 2022. PMID: 36007299 Review.
Cited by
-
Real-world pharmacovigilance of ofatumumab in multiple sclerosis: a comprehensive FAERS data analysis.Front Pharmacol. 2025 Jan 23;15:1521726. doi: 10.3389/fphar.2024.1521726. eCollection 2024. Front Pharmacol. 2025. PMID: 39917326 Free PMC article.
-
NEK2 regulates B cell function and the severity of experimental autoimmune encephalomyelitis.J Neuroinflammation. 2025 Jun 6;22(1):152. doi: 10.1186/s12974-025-03472-w. J Neuroinflammation. 2025. PMID: 40481479 Free PMC article.
-
Real world effectiveness, persistence, tolerability, and safety of ofatumumab in clinical practice.Neurodegener Dis Manag. 2025 Feb;15(1):27-36. doi: 10.1080/17582024.2025.2452150. Epub 2025 Jan 21. Neurodegener Dis Manag. 2025. PMID: 39834277 Free PMC article.
-
Profile of Ofatumumab in the Treatment of Multiple Sclerosis: Design, Development and Place in Therapy.Drug Des Devel Ther. 2024 Dec 12;18:5985-5996. doi: 10.2147/DDDT.S315174. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39687682 Free PMC article. Review.
-
Selective IgM Hypogammaglobulinemia and Multiple Sclerosis Treated with Natalizumab and Ofatumumab: A Case Report.J Pers Med. 2025 Apr 17;15(4):155. doi: 10.3390/jpm15040155. J Pers Med. 2025. PMID: 40278334 Free PMC article.
References
-
- US Food and Drug Administration. KESIMPTA® (ofatumumab) prescribing information, https://www.novartis.us/sites/www.novartis.us/files/kesimpta.pdf (accessed April 2022).
-
- European Medicines Agency. Kesimpta SmPC, https://www.ema.europa.eu/en/documents/product-information/kesimpta-epar.... (accessed April 2022).
-
- Hauser SL, Bar-Or A, Cohen JA. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med 2020; 383: 546–557. - PubMed
-
- Novartis Press Release. FDA approves Novartis Kesimpta® (ofatumumab), the first and only self-administered, targeted B-cell therapy for patients with relapsing multiple sclerosis, https://www.novartis.com/news/media-releases/fda-approves-novartis-kesim... (accessed March 2023).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

