TRiC/CCT chaperonin is required for the folding and inhibitory effect of WDTC1 on adipogenesis
- PMID: 37691821
- PMCID: PMC10483223
- DOI: 10.3389/fcell.2023.1225628
TRiC/CCT chaperonin is required for the folding and inhibitory effect of WDTC1 on adipogenesis
Abstract
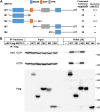
Obesity has become a global pandemic. WDTC1 is a WD40-containing protein that functions as an anti-obesity factor. WDTC1 inhibits adipogenesis by working as an adaptor of the CUL4-DDB1 E3 ligase complex. It remains unclear about how WDTC1 is regulated. Here, we show that the TRiC/CCT functions as a chaperone to facilitate the protein folding of WDTC1 and proper function in adipogenesis. Through tandem purification, we identified the molecular chaperone TRiC/CCT as WDTC1-interacting proteins. WDTC1 bound the TRiC/CCT through its ADP domain, and the TRiC/CCT recognized WDTC1 through the CCT5 subunit. Disruption of the TRiC/CCT by knocking down CCT1 or CCT5 led to misfolding and lysosomal degradation of WDTC1. Furthermore, the knockdown of CCT1 or CCT5 eliminated the inhibitory effect of WDTC1 on adipogenesis. Our studies uncovered a critical role of the TRiC/CCT in the folding of WDTC1 and expanded our knowledge on the regulation of adipogenesis.
Keywords: TRiC/CCT; WDTC1; adipogenesis; obesity; protein folding.
Copyright © 2023 Tang, Cen, Yao, Yin, Weng, Zhao and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Co-expression of CCT subunits hints at TRiC assembly.Cell Stress Chaperones. 2019 Nov;24(6):1055-1065. doi: 10.1007/s12192-019-01028-5. Epub 2019 Aug 13. Cell Stress Chaperones. 2019. PMID: 31410727 Free PMC article.
-
The molecular chaperone TRiC/CCT binds to the Trp-Asp 40 (WD40) repeat protein WDR68 and promotes its folding, protein kinase DYRK1A binding, and nuclear accumulation.J Biol Chem. 2014 Nov 28;289(48):33320-32. doi: 10.1074/jbc.M114.586115. Epub 2014 Oct 22. J Biol Chem. 2014. PMID: 25342745 Free PMC article.
-
Domain-specific folding of the tandem β-propeller protein Coronin 7 (Coro7) by CCT/TRiC.bioRxiv [Preprint]. 2025 Mar 11:2025.03.11.642617. doi: 10.1101/2025.03.11.642617. bioRxiv. 2025. PMID: 40161770 Free PMC article. Preprint.
-
The structural basis of eukaryotic chaperonin TRiC/CCT: Action and folding.Mol Cells. 2024 Mar;47(3):100012. doi: 10.1016/j.mocell.2024.100012. Epub 2024 Jan 26. Mol Cells. 2024. PMID: 38280673 Free PMC article. Review.
-
TRiC/CCT Chaperonin: Structure and Function.Subcell Biochem. 2019;93:625-654. doi: 10.1007/978-3-030-28151-9_19. Subcell Biochem. 2019. PMID: 31939165 Review.
References
-
- Galgani J. E., Kelley D. E., Albu J. B., Krakoff J., Smith S. R., Bray G. A., et al. (2013). Adipose tissue expression of adipose (WDTC1) gene is associated with lower fat mass and enhanced insulin sensitivity in humans. Obes. (Silver Spring) 21 (11), 2244–2248. 10.1002/oby.20371 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

