A tightly controlled gene induction system that contributes to the study of lethal gene function in Streptococcus mutans
- PMID: 37691880
- PMCID: PMC10486305
- DOI: 10.1080/20002297.2023.2253675
A tightly controlled gene induction system that contributes to the study of lethal gene function in Streptococcus mutans
Abstract
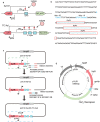
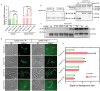
Effective control of gene expression is crucial for understanding gene function in both eukaryotic and prokaryotic cells. While several inducible gene expression systems have been reported in Streptococcus mutans, a conditional pathogen that causes dental caries, the significant non-inducible basal expression in these systems seriously limits their utility, especially when studying lethal gene functions and molecular mechanisms. We introduce a tightly controlled xylose-inducible gene expression system, TC-Xyl, for Streptococcus mutans. Western blot results and fluorescence microscopy analysis indicate that TC-Xyl exhibits an extremely low non-inducible basal expression level and a sufficiently high expression level post-induction. Further, by constructing a mutation in which the only source FtsZ is under the control of TC-Xyl, we preliminarily explored the function of the ftsz gene. We found that FtsZ depletion is lethal to Streptococcus mutans, resulting in abnormal round cell shape and mini cell formation, suggesting FtsZ's role in maintaining cell shape stability.
Keywords: FtsZ; Gene induction system; Streptococcus mutans; lethal gene function; xylose-induction.
© 2023 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
LinkOut - more resources
Full Text Sources
