Cancer immunotherapies: advances and bottlenecks
- PMID: 37691932
- PMCID: PMC10484345
- DOI: 10.3389/fimmu.2023.1212476
Cancer immunotherapies: advances and bottlenecks
Abstract
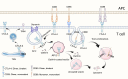
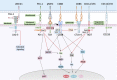
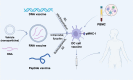
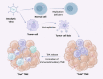
Immunotherapy has ushered in a new era in cancer treatment, and cancer immunotherapy continues to be rejuvenated. The clinical goal of cancer immunotherapy is to prime host immune system to provide passive or active immunity against malignant tumors. Tumor infiltrating leukocytes (TILs) play an immunomodulatory role in tumor microenvironment (TME) which is closely related to immune escape of tumor cells, thus influence tumor progress. Several cancer immunotherapies, include immune checkpoint inhibitors (ICIs), cancer vaccine, adoptive cell transfer (ACT), have shown great efficacy and promise. In this review, we will summarize the recent research advances in tumor immunotherapy, including the molecular mechanisms and clinical effects as well as limitations of immunotherapy.
Keywords: cancer immunotherapy; immune checkpoint blockade; neoadjuvant immunotherapy; tumor microenvironment.
Copyright © 2023 Rui, Zhou and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

