BNT162b2 COVID-19 vaccination in children alters cytokine responses to heterologous pathogens and Toll-like receptor agonists
- PMID: 37691937
- PMCID: PMC10485613
- DOI: 10.3389/fimmu.2023.1242380
BNT162b2 COVID-19 vaccination in children alters cytokine responses to heterologous pathogens and Toll-like receptor agonists
Abstract
Background: Vaccines can have beneficial off-target (heterologous) effects that alter immune responses to, and protect against, unrelated infections. The heterologous effects of COVID-19 vaccines have not been investigated in children.
Aim: To investigate heterologous and specific immunological effects of BNT162b2 COVID-19 vaccination in children.
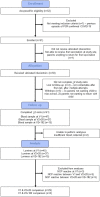
Methods: A whole blood stimulation assay was used to investigate in vitro cytokine responses to heterologous stimulants (killed pathogens, Toll-like receptor ligands) and SARS-CoV-2 antigens. Samples from 29 children, aged 5-11 years, before and 28 days after a second BNT162b2 vaccination were analysed (V2 + 28). Samples from eight children were analysed six months after BNT162b2 vaccination.
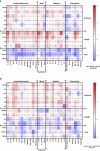
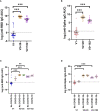
Results: At V2 + 28, interferon-γ and monocyte chemoattractant protein-1 responses to S. aureus, E. coli, L. monocytogenes, BCG vaccine, H. influenzae, hepatitis B antigen, poly(I:C) and R848 stimulations were decreased compared to pre-vaccination. For most of these heterologous stimulants, IL-6, IL-15 and IL-17 responses were also decreased. There were sustained decreases in cytokine responses to viral, but not bacterial, stimulants six months after BNT162b2 vaccination. Cytokine responses to irradiated SARS-CoV-2, and spike glycoprotein subunits (S1 and S2) were increased at V2 + 28 for most cytokines and remained higher than pre-vaccination responses 6 months after BNT162b2 vaccination for irradiated SARS-CoV-2 and S1. There was no correlation between BNT162b2 vaccination-induced anti-SARS-CoV2-receptor binding domain IgG antibody titre at V2 + 28 and cytokine responses.
Conclusions: BNT162b2 vaccination in children alters cytokine responses to heterologous stimulants, particularly one month after vaccination. This study is the first to report the immunological heterologous effects of COVID-19 vaccination in children.
Keywords: BNT162b2; SARS-CoV-2; heterologous; immunisation; innate immunity; mRNA vaccination; off-target effects; paediatric vaccination.
Copyright © 2023 Noé, Dang, Axelrad, Burrell, Germano, Elia, Burgner, Perrett, Curtis and Messina.
Conflict of interest statement
KP has received research grants from Aravax, DBV Technologies, Novartis and Siolta and consultant fees from Aravax, paid to their institution, outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

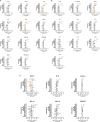
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

