Phenotypical changes of hematopoietic stem and progenitor cells in COVID-19 patients: Correlation with disease status
- PMID: 37692025
- PMCID: PMC10485691
- DOI: 10.5114/ceji.2023.129981
Phenotypical changes of hematopoietic stem and progenitor cells in COVID-19 patients: Correlation with disease status
Abstract
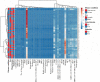
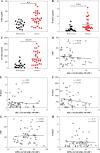
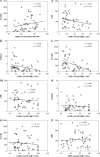
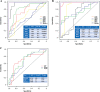
Hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) play a crucial role in the context of viral infections and their associated diseases. The link between HSCs and HPCs and disease status in COVID-19 patients is largely unknown. This study aimed to monitor the kinetics and contributions of HSCs and HPCs in severe and non-severe COVID-19 patients and to evaluate their diagnostic performance in differentiating between healthy and COVID-19 patients as well as severe and non-severe cases. Peripheral blood (PB) samples were collected from 48 COVID-19 patients, 16 recovered, and 27 healthy controls and subjected to deep flow cytometric analysis to determine HSCs and progenitor cells. Their diagnostic value and correlation with C-reactive protein (CRP), D-dimer, and ferritin levels were determined. The percentages of HSCs and common myeloid progenitors (CMPs) declined significantly, while the percentage of multipotent progenitors (MPPs) increased significantly in COVID-19 patients. There were no significant differences in the percentages of megakaryocyte-erythroid progenitors (MEPs) and granulocyte-macrophage progenitors (GMPs) between all groups. Severe COVID-19 patients had a significantly low percentage of HSCs, CMPs, and GMPs compared to non-severe cases. Contrarily, the levels of CRP, D-dimer, and ferritin increased significantly in severe COVID-19 patients. MPPs and CMPs showed excellent diagnostic performance in distinguishing COVID-19 patients from healthy controls and severe from non-severe COVID-19 patients, respectively. Collectively, our study indicated that hematopoietic stem and progenitor cells are significantly altered by COVID-19 and could be used as therapeutic targets and diagnostic biomarkers for severe COVID-19.
Keywords: COVID-19; SARS-CoV-2; hematopoietic progenitor cells (HPCs); hematopoietic stem cells (HSCs).
Copyright © 2023 Termedia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Sansonno D, Lotesoriere C, Cornacchiulo V, et al. . (1998): Hepatitis C virus infection involves CD34(+) hematopoietic progenitor cells in hepatitis C virus chronic carriers. Blood 92: 3328-3337. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
