Pyramiding of transcription factor, PgHSF4, and stress-responsive genes of p68, Pg47, and PsAKR1 impart multiple abiotic stress tolerance in rice (Oryza sativa L.)
- PMID: 37692421
- PMCID: PMC10492517
- DOI: 10.3389/fpls.2023.1233248
Pyramiding of transcription factor, PgHSF4, and stress-responsive genes of p68, Pg47, and PsAKR1 impart multiple abiotic stress tolerance in rice (Oryza sativa L.)
Abstract
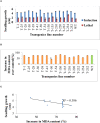
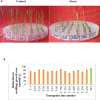
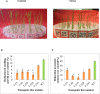
Abiotic stresses such as drought, salinity, and heat stress significantly affect rice crop growth and production. Under uncertain climatic conditions, the concurrent multiple abiotic stresses at different stages of rice production became a major challenge for agriculture. Hence, improving rice's multiple abiotic stress tolerance is essential to overcome unprecedented challenges under adverse environmental conditions. A significant challenge for rice breeding programs in improving abiotic stress tolerance involves multiple traits and their complexity. Multiple traits must be targeted to improve multiple stress tolerance in rice and uncover the mechanisms. With this hypothesis, in the present study gene stacking approach is used to integrate multiple traits involved in stress tolerance. The multigene transgenics co-expressing Pennisetum glaucum 47 (Pg47), Pea 68 (p68), Pennisetum glaucum Heat Shock Factor 4(PgHSF4), and Pseudomonas Aldo Keto Reductase 1 (PsAKR1) genes in the rice genotype (AC39020) were developed using the in-planta transformation method. The promising transgenic lines maintained higher yields under semi-irrigated aerobic cultivation (moisture stress). These 15 promising transgenic rice seedlings showed improved shoot and root growth traits under salinity, accelerating aging, temperature, and oxidative stress. They showed better physiological characteristics, such as chlorophyll content, membrane stability, and lower accumulation of reactive oxygen species, under multiple abiotic stresses than wild-type. Enhanced expression of transgenes and other stress-responsive downstream genes such as HSP70, SOD, APX, SOS, PP2C, and P5CS in transgenic lines suggest the possible molecular mechanism for imparting the abiotic stress tolerance. This study proved that multiple genes stacking as a novel strategy induce several mechanisms and responsible traits to overcome multiple abiotic stresses. This multigene combination can potentially improve tolerance to multiple abiotic stress conditions and pave the way for developing climate-resilient crops.
Keywords: abiotic stress; gene stacking; mechanism; multiple genes; stress tolerance; trait; transgenics.
Copyright © 2023 Sheela, Vennapusa, Melmaiee, Prasad and Reddy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Ali A., Beena R., Manikanta C. ,. L. N., Swapna A., Soni K. B., Viji M. M. (2022). Molecular characterization and varietal identification for multiple abiotic stress tolerance in rice (Oryza sativa L.). Oryza-An Int. J. Rice 59 (1), 140–150. doi: 10.35709/ory.2022.59.1.7 - DOI
-
- Balasubramanium V., Maheshwari M. (1989). Comparison of physiological response of pearl millet and sorghum to water stresses. Proc. Indian Acad. Sci. 99, 517–522. doi: 10.1007/BF03053420 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

