Chloroplast magnesium transporters play essential but differential roles in maintaining magnesium homeostasis
- PMID: 37692441
- PMCID: PMC10484576
- DOI: 10.3389/fpls.2023.1221436
Chloroplast magnesium transporters play essential but differential roles in maintaining magnesium homeostasis
Abstract
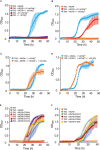
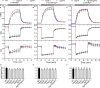
Magnesium (Mg2+) is essential for photosynthesis in the chloroplasts of land plants and algae. Being the central ion of chlorophyll, cofactor and activator of many photosynthetic enzymes including RuBisCO, magnesium-deficient plants may suffer from leaf chlorosis symptoms and retarded growth. Therefore, the chloroplast Mg2+ concentration is tightly controlled by magnesium transport proteins. Recently, three different transporters from two distinct families have been identified in the chloroplast inner envelope of the model plant Arabidopsis thaliana: MGT10, MGR8, and MGR9. Here, we assess the individual roles of these three proteins in maintaining chloroplast Mg2+ homeostasis and regulating photosynthesis, and if their role is conserved in the model green alga Chlamydomonas reinhardtii. Phylogenetic analysis and heterologous expression revealed that the CorC-like MGR8 and MGR9 transport Mg2+ by a different mechanism than the CorA-like MGT10. MGR8 and MGT10 genes are highest expressed in leaves, indicating a function in chloroplast Mg2+ transport. MGR9 is important for chloroplast function and plant adaptation in conditions of deficiency or excess of Mg2+. Transmission electron microscopy indicated that MGT10 plays a differential role in thylakoid stacking than MGR8 and MGR9. Furthermore, we report that MGR8, MGR9, and MGT10 are involved in building up the pH gradient across the thylakoid membrane and activating photoprotection in conditions of excess light, however the mechanism has not been resolved yet. While there are no chloroplast MGR-like transporters in Chlamydomonas, we show that MRS4 is a homolog of MGT10, that is required for photosynthesis and cell growth. Taken together, our findings reveal that the studied Mg2+ transporters play essential but differential roles in maintaining chloroplast Mg2+ homeostasis.
Keywords: Arabidopsis thaliana; Chlamydomonas reinhardtii; chlorophyll fluorescence; chloroplast; magnesium homeostasis; magnesium transporter; photosynthesis.
Copyright © 2023 Dukic, van Maldegem, Shaikh, Fukuda, Töpel, Solymosi, Hellsten, Hansen, Husted, Higgins, Sano, Ishijima and Spetea.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Böszörményi A., Dobi A., Skribanek A., Pávai M., Solymosi K. (2020). The effect of light on plastid differentiation, chlorophyll biosynthesis, and essential oil composition in rosemary (Rosmarinus officinalis) leaves and cotyledons. Front. Plant Sci. 11, 196. doi: 10.3389/fpls.2020.00196 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

