Fantastic voyage: The journey of NLRP3 inflammasome activation
- PMID: 37692521
- PMCID: PMC10491867
- DOI: 10.1016/j.gendis.2023.01.009
Fantastic voyage: The journey of NLRP3 inflammasome activation
Abstract
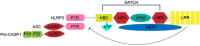
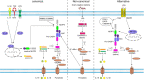
NLRP3 inflammasome, an intracellular multiprotein complex, can be activated by a range of pathogenic microbes or endogenous hazardous chemicals. Its activation results in the release of cytokines such as IL-1β and IL-18, as well as Gasdermin D which eventually causes pyroptosis. The activation of NLRP3 inflammasome is under strict control and regulation by numerous pathways and mechanisms. Its excessive activation can lead to a persistent inflammatory response, which is linked to the onset and progression of severe illnesses. Recent studies have revealed that the subcellular localization of NLRP3 changes significantly during the activation process. In this review, we review the current understanding of the molecular mechanism of NLRP3 inflammasome activation, focusing on the subcellular localization of NLRP3 and the associated regulatory mechanisms. We aim to provide a comprehensive understanding of the dynamic transportation, activation, and degradation processes of NLRP3.
Keywords: Inflammasome activation; Innate immunity; NLRP3; NLRP3 inflammasome; Subcellular localization.
© 2023 Chongqing Medical University. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

Similar articles
-
The zebrafish NLRP3 inflammasome has functional roles in ASC-dependent interleukin-1β maturation and gasdermin E-mediated pyroptosis.J Biol Chem. 2020 Jan 24;295(4):1120-1141. doi: 10.1074/jbc.RA119.011751. Epub 2019 Dec 18. J Biol Chem. 2020. PMID: 31852739 Free PMC article.
-
The double sides of NLRP3 inflammasome activation in sepsis.Clin Sci (Lond). 2023 Mar 15;137(5):333-351. doi: 10.1042/CS20220556. Clin Sci (Lond). 2023. PMID: 36856019 Review.
-
The NLRP3 inflammasome: molecular activation and regulation to therapeutics.Nat Rev Immunol. 2019 Aug;19(8):477-489. doi: 10.1038/s41577-019-0165-0. Nat Rev Immunol. 2019. PMID: 31036962 Free PMC article. Review.
-
Glucose regulates hypoxia-induced NLRP3 inflammasome activation in macrophages.J Cell Physiol. 2020 Oct;235(10):7554-7566. doi: 10.1002/jcp.29659. Epub 2020 Mar 1. J Cell Physiol. 2020. PMID: 32115713
-
Enterococcus Faecalis activates NLRP3 inflammasomes leading to increased interleukin-1 beta secretion and pyroptosis of THP-1 macrophages.Microb Pathog. 2021 May;154:104761. doi: 10.1016/j.micpath.2021.104761. Epub 2021 Jan 29. Microb Pathog. 2021. PMID: 33524566
Cited by
-
Cardio-Rheumatic Diseases: Inflammasomes Behaving Badly.Int J Mol Sci. 2025 Apr 9;26(8):3520. doi: 10.3390/ijms26083520. Int J Mol Sci. 2025. PMID: 40331999 Free PMC article. Review.
-
Pyroptosis: A spoiler of peaceful coexistence between cells in degenerative bone and joint diseases.J Adv Res. 2025 May;71:227-262. doi: 10.1016/j.jare.2024.06.010. Epub 2024 Jun 13. J Adv Res. 2025. PMID: 38876191 Free PMC article. Review.
-
The stress-responsive cytotoxic effect of diesel exhaust particles on lymphatic endothelial cells.Sci Rep. 2024 May 7;14(1):10503. doi: 10.1038/s41598-024-61255-4. Sci Rep. 2024. PMID: 38714844 Free PMC article.
-
LINC01013 reverses bisphosphonate-impaired osteogenic differentiation of JBMMSCs by regulating intracellular translocation of ILF3.Stem Cell Res Ther. 2025 Jun 23;16(1):319. doi: 10.1186/s13287-025-04467-3. Stem Cell Res Ther. 2025. PMID: 40551247 Free PMC article.
-
NLRP3 Inflammasome in Vascular Dementia: Regulatory Mechanisms, Functions, and Therapeutic Implications: A Comprehensive Review.CNS Neurosci Ther. 2025 May;31(5):e70403. doi: 10.1111/cns.70403. CNS Neurosci Ther. 2025. PMID: 40326096 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

