A Review of 10-Year Survivability of Immunotherapy in the Management of Colon Cancer
- PMID: 37692610
- PMCID: PMC10485874
- DOI: 10.7759/cureus.43189
A Review of 10-Year Survivability of Immunotherapy in the Management of Colon Cancer
Abstract
Colon cancer is one of the most common cancers in the United States of America. In addition to conventional treatment approaches such as surgery, chemotherapy, and radiation for colorectal cancer, immunotherapy has gained recognition over the past few years. However, its effectiveness in colorectal cancer treatment is controversial. Our study investigates the survival and progression-free rates of immunotherapy for different types of colorectal cancer over the last 10 years. We conducted literature reviews from various clinical trials and research studies to evaluate immunotherapy's role in colorectal cancer treatment. We also investigated how it affects clinical outcomes. We discovered a range of effective immunotherapy approaches targeting various growth factors and signaling pathways. These modalities include monoclonal antibodies aimed at growth factors such as epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), human epidermal growth factor receptor 2 (HER2), and downstream signaling pathways such as mitogen-activated protein kinase (MAPK), kirsten rat sarcoma viral oncogene (KRAS), B-raf proto-oncogene, serine/threonine kinase (BRAF), and phosphatase and tensin homolog (PTEN). Additionally, we identified immune checkpoint inhibitors, such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibitors and programmed cell death ligand 1 (PD-L1) inhibitors, as well as target therapy and adoptive cell therapy as promising immunotherapeutic options. Nevertheless, the application of immunotherapy remains highly limited due to various factors influencing survival and progression-free rates, including tumor microenvironment, microsatellite instability, immune checkpoint expression, and gut microbiome. Additionally, its effectiveness is restricted to a small subgroup of patients, accompanied by side effects and the development of drug resistance mechanisms. To unlock its full potential, further clinical trials and research on molecular pathways in colorectal cancer are imperative. This will ultimately enhance drug discovery success and lead to more effective clinical management approaches.
Keywords: colon adenocarcinoma; colon gist; colon lymphoma; immunotherapy; progression-free rates; survival rates.
Copyright © 2023, Okoye et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

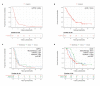
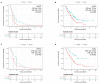
References
-
- American Cancer Society. Cancer Facts & Figures 2021. [ May; 2023 ]. 2021. https://bcan.org/wp-content/uploads/2021/01/cancer-facts-and-figures-202... https://bcan.org/wp-content/uploads/2021/01/cancer-facts-and-figures-202...
-
- Basic Information About Colorectal Cancer. [ May; 2023 ]. 2020. https://www.cdc.gov/cancer/colorectal/basic_info/index.htm https://www.cdc.gov/cancer/colorectal/basic_info/index.htm
-
- National Cancer Institute. National Cancer Institute. Colon Cancer Treatment (PDQ®)-Health Professional Version. [ May; 2023 ]. 2019. https://www.cancer.gov/types/colorectal/hp/colon-treatment-pdq https://www.cancer.gov/types/colorectal/hp/colon-treatment-pdq
-
- American Society of Clinical Oncology. Colorectal Cancer: Statistics. [ May; 2023 ]. 2019. https://www.cancer.net/cancer-types/colorectal-cancer/statistics https://www.cancer.net/cancer-types/colorectal-cancer/statistics
-
- American Cancer Society. Colorectal cancer guideline | How often to have screening tests. [ May; 2023 ]. 2019. https://www.cancer.org/cancer/types/colon-rectal-cancer/detection-diagno... https://www.cancer.org/cancer/types/colon-rectal-cancer/detection-diagno...
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
