Shaping the "hot" immunogenic tumor microenvironment by nanoparticles co-delivering oncolytic peptide and TGF-β1 siRNA for boosting checkpoint blockade therapy
- PMID: 37693065
- PMCID: PMC10487304
- DOI: 10.1002/btm2.10392
Shaping the "hot" immunogenic tumor microenvironment by nanoparticles co-delivering oncolytic peptide and TGF-β1 siRNA for boosting checkpoint blockade therapy
Abstract
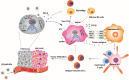
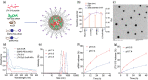
Induction of potent immune responses toward tumors remains challenging in cancer immunotherapy, in which it only showed benefits in a minority of patients with "hot" tumors, which possess pre-existing effector immune cells within the tumor. In this study, we proposed a nanoparticle-based strategy to fire up the "cold" tumor by upregulating the components associated with T and NK cell recruitment and activation and suppressing TGF-β1 secretion by tumor cells. Specifically, LTX-315, a first-in-class oncolytic cationic peptide, and TGF-β1 siRNA were co-entrapped in a polymer-lipid hybrid nanoparticle comprising PLGA, DSPE-mPEG, and DSPE-PEG-conjugated with cRGD peptide (LTX/siR-NPs). The LTX/siR-NPs showed significant inhibition of TGF-β1 expression, induction of type I interferon release, and triggering immunogenic cell death (ICD) in treated tumor cells, indicated via the increased levels of danger molecules, an in vitro setting. The in vivo data showed that the LTX/siR-NPs could effectively protect the LTX-315 peptide from degradation in serum, which highly accumulated in tumor tissue. Consequently, the LTX/siR-NPs robustly suppressed TGF-β1 production by tumor cells and created an immunologically active tumor with high infiltration of antitumor effector immune cells. As a result, the combination of LTX/siR-NP treatment with NKG2A checkpoint inhibitor therapy remarkably increased numbers of CD8+NKG2D+ and NK1.1+NKG2D+ within tumor masses, and importantly, inhibited the tumor growth and prolonged survival rate of treated mice. Taken together, this study suggests the potential of the LTX/siR-NPs for inflaming the "cold" tumor for potentiating the efficacy of cancer immunotherapy.
Keywords: LTX‐315; cancer immunotherapy; hybrid nanoparticle; tumor microenvironment.
© 2022 The Authors. Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of the American Institute of Chemical Engineers.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

