Partial decellularization eliminates immunogenicity in tracheal allografts
- PMID: 37693070
- PMCID: PMC10487308
- DOI: 10.1002/btm2.10525
Partial decellularization eliminates immunogenicity in tracheal allografts
Abstract
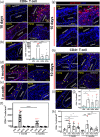
There is currently no suitable autologous tissue to bridge large tracheal defects. As a result, no standard of care exists for long-segment tracheal reconstruction. Tissue engineering has the potential to create a scaffold from allografts or xenografts that can support neotissue regeneration identical to the native trachea. Recent advances in tissue engineering have led to the idea of partial decellularization that allows for the creation of tracheal scaffolds that supports tracheal epithelial formation while preserving mechanical properties. However, the ability of partial decellularization to eliminate graft immunogenicity remains unknown, and understanding the immunogenic properties of partially decellularized tracheal grafts (PDTG) is a critical step toward clinical translation. Here, we determined that tracheal allograft immunogenicity results in epithelial cell sloughing and replacement with dysplastic columnar epithelium and that partial decellularization creates grafts that are able to support an epithelium without histologic signs of rejection. Moreover, allograft implantation elicits CD8+ T-cell infiltration, a mediator of rejection, while PDTG did not. Hence, we establish that partial decellularization eliminates allograft immunogenicity while creating a scaffold for implantation that can support spatially appropriate airway regeneration.
Keywords: decellularization; immunogenicity; orthotopic tracheal transplantation; regenerative medicine; tissue‐engineered tracheal graft.
© 2023 The Authors. Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of The American Institute of Chemical Engineers.
Conflict of interest statement
The authors have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

