EUS-guided versus percutaneous liver biopsy: A prospective randomized clinical trial
- PMID: 37693114
- PMCID: PMC10437149
- DOI: 10.1097/eus.0000000000000010
EUS-guided versus percutaneous liver biopsy: A prospective randomized clinical trial
Abstract
Background and objectives: Prospective studies comparing EUS-guided liver biopsy (EUS-LB) to percutaneous LB (PC-LB) are scarce. We compared the efficacy and safety of EUS-LB with those of PC-LB in a prospective randomized clinical trial.
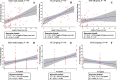
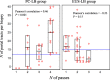
Methods: Between 2020 and 2021, patients were enrolled and randomized (1:1 ratio). The primary outcome was defined as the proportion of patients with ≥11 complete portal tracts (CPTs). The sample size (n = 80) was calculated based on the assumption that 60% of those in the EUS-LB and 90% of those in the PC-LB group will have LB with ≥11 CPTs. The secondary outcomes included proportion of patients in whom a diagnosis was established, number of CPTs, pain severity (Numeric Rating Scale-Pain Intensity), duration of hospital stay, and adverse events.
Results: Eighty patients were enrolled (median age, 53 years); 67.5% were female. Sixty percent of those in the EUS-LB and 75.0% of those in the PC-LB group met the primary outcome (P = 0.232). The median number of CPTs was higher in the PC-LB (17 vs 13; P = 0.031). The proportion of patients in whom a diagnosis was established was similar between the groups (92.5% [EUS-LB] vs 95.0% [PC-LB]; P = 1.0). Patients in the EUS-LB group had less pain severity (median Numeric Rating Scale-Pain Intensity, 2.0 vs 3.0; P = 0.003) and shorter hospital stay (2.0 vs 4.0 hours; P < 0.0001) compared with the PC-LB group. No patient experienced a serious adverse event.
Conclusions: EUS-guided liver biopsy was safe, effective, better tolerated, and associated with a shorter hospital stay.
Keywords: Complete portal tracts; EUS; Efficacy; Fine needle biopsy; Histological diagnosis; Liver biopsy; Percutaneous; Safety.
Copyright © 2023 The Author(s). Published by Wolters Kluwer on behalf of Scholar Media Publishing.
Conflict of interest statement
All authors have no conflict of interest to declare.
Figures
Similar articles
-
The efficacy and safety of endoscopic ultrasound-guided liver biopsy versus percutaneous liver biopsy in patients with chronic liver disease: a retrospective single-center study.J Ultrasound. 2020 Jun;23(2):157-167. doi: 10.1007/s40477-020-00436-z. Epub 2020 Mar 5. J Ultrasound. 2020. PMID: 32141043 Free PMC article.
-
Diagnostic Yield of Endoscopic Ultrasound-Guided Liver Biopsy in Comparison to Percutaneous Liver Biopsy: A Two-Center Experience.Cancers (Basel). 2021 Jun 19;13(12):3062. doi: 10.3390/cancers13123062. Cancers (Basel). 2021. PMID: 34205389 Free PMC article.
-
EUS-guided fine-needle liver biopsy in pediatric patients using a modified technique with one-pass, one-actuation wet suction.Rev Esp Enferm Dig. 2022 Oct;114(10):575-579. doi: 10.17235/reed.2022.8503/2021. Rev Esp Enferm Dig. 2022. PMID: 35040332
-
Endoscopic ultrasound-guided versus percutaneous liver biopsy: a systematic review and meta-analysis of randomized controlled trials.Endoscopy. 2025 Jan;57(1):41-48. doi: 10.1055/a-2368-4608. Epub 2024 Aug 28. Endoscopy. 2025. PMID: 39197465
-
Diagnostic Yield of Endoscopic Ultrasound-Guided Liver Biopsy in Comparison to Percutaneous Liver Biopsy: A Meta-Analysis of Randomized Controlled Trials and Trial Sequential Analysis.Diagnostics (Basel). 2024 Jun 12;14(12):1238. doi: 10.3390/diagnostics14121238. Diagnostics (Basel). 2024. PMID: 38928653 Free PMC article. Review.
Cited by
-
EUS-guided versus percutaneous liver biopsy: A comprehensive review and meta-analysis of outcomes.Endosc Ultrasound. 2023 Mar-Apr;12(2):171-180. doi: 10.4103/EUS-D-21-00268. Endosc Ultrasound. 2023. PMID: 36204798 Free PMC article. Review.
-
Endoscopic ultrasound-guided tissue acquisition for focal liver lesions can be safely performed in patients with ascites.Endosc Int Open. 2024 Nov 18;12(11):E1309-E1314. doi: 10.1055/a-2427-2427. eCollection 2024 Nov. Endosc Int Open. 2024. PMID: 39559415 Free PMC article.
-
Endo-hepatology: Why should we do endoscopic ultrasound-guided interventions to the liver that we could do through the skin?World J Gastroenterol. 2024 Oct 28;30(40):4333-4338. doi: 10.3748/wjg.v30.i40.4333. World J Gastroenterol. 2024. PMID: 39494095 Free PMC article.
-
Remnant cholesterol, a potential risk factor of metabolic dysfunction-associated fatty liver disease.Nutr Metab (Lond). 2025 Feb 18;22(1):13. doi: 10.1186/s12986-025-00898-0. Nutr Metab (Lond). 2025. PMID: 39966919 Free PMC article.
-
Association of dietary quality and mortality in the non-alcoholic fatty liver disease and advanced fibrosis populations: NHANES 2005-2018.Front Nutr. 2025 Jan 23;12:1507342. doi: 10.3389/fnut.2025.1507342. eCollection 2025. Front Nutr. 2025. PMID: 39917744 Free PMC article.
References
-
- Zeng K Jiang Z Yang J, et al. . Role of endoscopic ultrasound–guided liver biopsy: a meta-analysis. Scand J Gastroenterol 2022;57:545–557. - PubMed
-
- Baran B Kale S Patil P, et al. . Endoscopic ultrasound–guided parenchymal liver biopsy: a systematic review and meta-analysis. Surg Endosc 2021;35:5546–5557. - PubMed
-
- Mohan BP Shakhatreh M Garg R, et al. . Efficacy and safety of EUS-guided liver biopsy: a systematic review and meta-analysis. Gastrointest Endosc 2019;89:238–246.e3. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

