This is a preprint.
Cryptosporidium infection of human small intestinal epithelial cells induces type III interferon and impairs infectivity of Rotavirus
- PMID: 37693422
- PMCID: PMC10491271
- DOI: 10.1101/2023.08.30.555581
Cryptosporidium infection of human small intestinal epithelial cells induces type III interferon and impairs infectivity of Rotavirus
Update in
-
Cryptosporidium infection of human small intestinal epithelial cells induces type III interferon and impairs infectivity of Rotavirus.Gut Microbes. 2024 Jan-Dec;16(1):2297897. doi: 10.1080/19490976.2023.2297897. Epub 2024 Jan 8. Gut Microbes. 2024. PMID: 38189373 Free PMC article.
Abstract
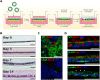
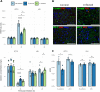
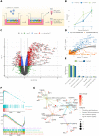
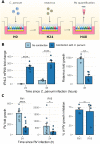
Cryptosporidiosis is a major cause of severe diarrheal disease in infants from resource poor settings. The majority of infections are caused by the human-specific pathogen C. hominis and absence of in vitro growth platforms has limited our understanding of host-pathogen interactions and development of effective treatments. To address this problem, we developed a stem cell-derived culture system for C. hominis using human enterocytes differentiated under air-liquid interface (ALI) conditions. Human ALI cultures supported robust growth and complete development of C. hominis in vitro including all life cycle stages. C. hominis infection induced a strong interferon response from enterocytes, likely driven by an endogenous dsRNA virus in the parasite. Prior infection with Cryptosporidium induced type III IFN secretion and consequently blunted infection with Rotavirus, including live attenuated vaccine strains. The development of hALI provides a platform for further studies on human-specific pathogens, including clinically important coinfections that may alter vaccine efficacy.
Keywords: antiviral; co-infection; enteric infection; interferon; mucosal immunity; parasitology; vaccine.
Figures


References
-
- Feng Y, Ryan UM, Xiao L. Genetic Diversity and Population Structure of Cryptosporidium. Trends Parasitol. 2018;34(11):997–1011. - PubMed
-
- Kotloff KL, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. The Lancet. 2013;382(9888):209–222. - PubMed
-
- Kotloff KL. The Burden and Etiology of Diarrheal Illness in Developing Countries. Pediatr Clin North Am. 2017;64(4):799–814. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
