This is a preprint.
HCN channels sense temperature and determine heart rate responses to heat
- PMID: 37693513
- PMCID: PMC10491304
- DOI: 10.1101/2023.09.02.556046
HCN channels sense temperature and determine heart rate responses to heat
Update in
-
HCN4 channels sense temperature and determine heart rate responses to heat.Nat Commun. 2025 Mar 1;16(1):2102. doi: 10.1038/s41467-025-57358-9. Nat Commun. 2025. PMID: 40025061 Free PMC article.
Abstract
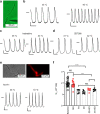
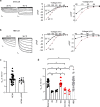
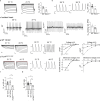
Heart rate increases with heat, [1-3] constituting a fundamental physiological relationship in vertebrates. Each normal heartbeat is initiated by an action potential generated in a sinoatrial nodal pacemaker cell. Pacemaker cells are enriched with hyperpolarization activated cyclic nucleotide-gated ion channels (HCN) that deliver cell membrane depolarizing inward current that triggers action potentials. HCN channel current increases due to cAMP binding, a mechanism coupling adrenergic tone to physiological 'fight or flight' heart rate acceleration. However, the mechanism(s) for heart rate response to thermal energy is unknown. We used thermodynamical and homology computational modeling, site-directed mutagenesis and mouse models to identify a concise motif on the S4-S5 linker of the cardiac pacemaker HCN4 channels (M407/Y409) that determines HCN4 current (If) and cardiac pacemaker cell responses to heat. This motif is required for heat sensing in cardiac pacemaker cells and in isolated hearts. In contrast, the cyclic nucleotide binding domain is not required for heat induced HCN4 current increases. However, a loss of function M407/Y409 motif mutation prevented normal heat and cAMP responses, suggesting that heat sensing machinery is essential for operating the cAMP allosteric pathway and is central to HCN4 modulation. The M407/Y409 motif is conserved across all HCN family members suggesting that HCN channels participate broadly in coupling heat to changes in cell membrane excitability.
Figures


References
-
- Kirschen G.W., et al., Relationship between body temperature and heart rate in adults and children: A local and national study. Am J Emerg Med, 2020. 38(5): p. 929–933. - PubMed
-
- Biel M., et al., Hyperpolarization-Activated Cation Channels: From Genes to Function. Physiological Reviews, 2009. 89(3): p. 847–885. - PubMed
-
- DiFrancesco D., Pacemaker mechanisms in cardiac tissue. Annual review of physiology, 1993. 55(1): p. 455–472. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
