This is a preprint.
Loss of finger control complexity and intrusion of flexor biases are dissociable in finger individuation impairment after stroke
- PMID: 37693573
- PMCID: PMC10491249
- DOI: 10.1101/2023.08.29.555444
Loss of finger control complexity and intrusion of flexor biases are dissociable in finger individuation impairment after stroke
Abstract
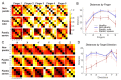
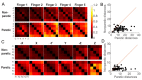
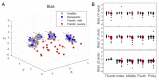
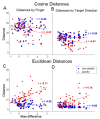
The ability to control each finger independently is an essential component of human hand dexterity. A common observation of hand function impairment after stroke is the loss of this finger individuation ability, often referred to as enslavement, i.e., the unwanted coactivation of non-intended fingers in individuated finger movements. In the previous literature, this impairment has been attributed to several factors, such as the loss of corticospinal drive, an intrusion of flexor synergy due to upregulations of the subcortical pathways, and/or biomechanical constraints. These factors may or may not be mutually exclusive and are often difficult to tease apart. It has also been suggested, based on a prevailing impression, that the intrusion of flexor synergy appears to be an exaggerated pattern of the involuntary coactivations of task-irrelevant fingers seen in a healthy hand, often referred to as a flexor bias. Most previous studies, however, were based on assessments of enslavement in a single dimension (i.e., finger flexion/extension) that coincide with the flexor bias, making it difficult to tease apart the other aforementioned factors. Here, we set out to closely examine the nature of individuated finger control and finger coactivation patterns in all dimensions. Using a novel measurement device and a 3D finger-individuation paradigm, we aim to tease apart the contributions of lower biomechanical, subcortical constraints, and top-down cortical control to these patterns in both healthy and stroke hands. For the first time, we assessed all five fingers' full capacity for individuation. Our results show that these patterns in the healthy and paretic hands present distinctly different shapes and magnitudes that are not influenced by biomechanical constraints. Those in the healthy hand presented larger angular distances that were dependent on top-down task goals, whereas those in the paretic hand presented larger Euclidean distances that arise from two dissociable factors: a loss of complexity in finger control and the dominance of an intrusion of flexor bias. These results suggest that finger individuation impairment after stroke is due to two dissociable factors: the loss of finger control complexity present in the healthy hand reflecting a top-down neural control strategy and an intrusion of flexor bias likely due to an upregulation of subcortical pathways. Our device and paradigm are demonstrated to be a promising tool to assess all aspects of the dexterous capacity of the hand.
Figures






References
-
- Bates Douglas, Machler Märtin, Bolker Ben, and Walker Steve. 2015. “Fitting Linear Mixed-Effects Models Using Lme4.” Journal of Statistical Software 67 (1): 1–48. 10.18637/jss.v067.i01. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
