This is a preprint.
Saccharomycotina yeasts defy longstanding macroecological patterns
- PMID: 37693602
- PMCID: PMC10491267
- DOI: 10.1101/2023.08.29.555417
Saccharomycotina yeasts defy longstanding macroecological patterns
Update in
-
Saccharomycotina yeasts defy long-standing macroecological patterns.Proc Natl Acad Sci U S A. 2024 Mar 5;121(10):e2316031121. doi: 10.1073/pnas.2316031121. Epub 2024 Feb 27. Proc Natl Acad Sci U S A. 2024. PMID: 38412132 Free PMC article.
Abstract
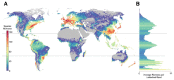
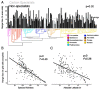
The Saccharomycotina yeasts ("yeasts" hereafter) are a fungal clade of scientific, economic, and medical significance. Yeasts are highly ecologically diverse, found across a broad range of environments in every biome and continent on earth1; however, little is known about what rules govern the macroecology of yeast species and their range limits in the wild2. Here, we trained machine learning models on 12,221 occurrence records and 96 environmental variables to infer global distribution maps for 186 yeast species (~15% of described species from 75% of orders) and to test environmental drivers of yeast biogeography and macroecology. We found that predicted yeast diversity hotspots occur in mixed montane forests in temperate climates. Diversity in vegetation type and topography were some of the greatest predictors of yeast species richness, suggesting that microhabitats and environmental clines are key to yeast diversification. We further found that range limits in yeasts are significantly influenced by carbon niche breadth and range overlap with other yeast species, with carbon specialists and species in high diversity environments exhibiting reduced geographic ranges. Finally, yeasts contravene many longstanding macroecological principles, including the latitudinal diversity gradient, temperature-dependent species richness, and latitude-dependent range size (Rapoport's rule). These results unveil how the environment governs the global diversity and distribution of species in the yeast subphylum. These high-resolution models of yeast species distributions will facilitate the prediction of economically relevant and emerging pathogenic species under current and future climate scenarios.
Conflict of interest statement
Conflict of Interest A.R. is a scientific consultant of LifeMine Therapeutics, Inc. The authors declare no other competing interests.
Figures


References
-
- Péter G., Takashima M. & Čadež N. in Yeasts in Natural Ecosystems: Ecology (eds. Buzzini P., Lachance M.-A. & Yurkov A.) 39–71 (Springer International Publishing, 2017). doi:10.1007/978-3-319-61575-2_2 - DOI
-
- Shen X.-X., Opulente D. A., Kominek J., Zhou X., Steenwyk J. L., Buh K. V., Haase M. A. B., Wisecaver J. H., Wang M., Doering D. T., Boudouris J. T., Schneider R. M., Langdon Q. K., Ohkuma M., Endoh R., Takashima M., Manabe R., Čadež N., Libkind D., Rosa C. A., DeVirgilio J., Hulfachor A. B., Groenewald M., Kurtzman C. P., Hittinger C. T. & Rokas A. Tempo and Mode of Genome Evolution in the Budding Yeast Subphylum. Cell 175, 1533–1545.e20 (2018). - PMC - PubMed
-
- Opulente D. A., LaBella A. L., Harrison M.-C., Wolters J. F., Liu C., Li Y., Kominek J., Steenwyk J. L., Stoneman H. R. & VanDenAvond J. Genomic and ecological factors shaping specialism and generalism across an entire subphylum. bioRxiv 2023.06. 19.545611 (2023).
-
- Starmer W. T. & Lachance M.-A. Yeast ecology. The yeasts 65–83 (2011).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
