Impact and effectiveness of Rotavin-M1 under conditions of routine use in two provinces in Vietnam, 2016-2021, an observational and case-control study
- PMID: 37693867
- PMCID: PMC10485664
- DOI: 10.1016/j.lanwpc.2023.100789
Impact and effectiveness of Rotavin-M1 under conditions of routine use in two provinces in Vietnam, 2016-2021, an observational and case-control study
Abstract
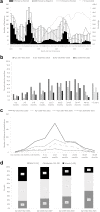
Background: Half of diarrhea hospitalizations in children aged <5 years in Vietnam are due to rotavirus. Following introduction of a locally developed and licensed oral rotavirus vaccine, Rotavin-M1, into the routine immunization program in two Vietnamese provinces, Nam Dinh and TT Hue, we describe changes in rotavirus positivity among children hospitalized for diarrhea and calculate vaccine effectiveness against moderate-to-severe rotavirus hospitalizations.
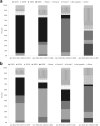
Methods: Active rotavirus surveillance among children <5 years began in December 2016 at sentinel hospitals in districts where rotavirus vaccine was introduced in December 2017. To estimate reductions in rotavirus detection, we calculated risk ratios comparing rotavirus positivity pre- and post-vaccine introduction. We used a test-negative case-control design to calculate vaccine effectiveness.
Findings: From December 2016 to May 2021, 7228 children <5 years hospitalized for diarrhea were enrolled. Following introduction, Rotavin-M1 coverage was 77% (1066/1377) in Nam Dinh and 42% (203/489) in TT Hue. In Nam Dinh, rotavirus positivity among children <5 years significantly declined by 40.6% (95% CI: 34.8%-45.8%) during the three-year post-vaccine introduction period. In TT Hue, no change in rotavirus positivity was observed. Among children aged 6-23 months, a 2-dose series of Rotavin-M1 was 57% (95% CI: 39%-70%) effective against moderate-to-severe rotavirus hospitalizations.
Interpretation: Higher vaccination coverage in Nam Dinh than TT Hue likely contributed to substantial declines in rotavirus positivity observed in Nam Dinh following rotavirus vaccine introduction. Robust vaccine effectiveness was observed through the second year of life. National rotavirus vaccine introduction with high coverage may have substantial impact on reducing rotavirus disease burden in Vietnam.
Funding: Bill and Melinda Gates Foundation.
Keywords: Rotavirus; Rotavirus vaccine; Vaccine effectiveness; Vietnam.
Conflict of interest statement
Huong Thuy Nguyen, Thao Phuong Thi Pham, Nguyen Dang Hien are the member of POLYVAC-Vietnam.
Figures


References
-
- Meeting of the strategic advisory group of experts on immunization, October 2009–conclusions and recommendations. Wkly Epidemiol Rec. 2009;84(50):517–532. - PubMed
-
- Rotavirus vaccines: WHO position paper–July 2021. Wkly Epidemiol Rec. 2021;96(28):301–319.
LinkOut - more resources
Full Text Sources

