Long-term proton pump inhibitors use and its association with premalignant gastric lesions: a systematic review and meta-analysis
- PMID: 37693896
- PMCID: PMC10492503
- DOI: 10.3389/fphar.2023.1244400
Long-term proton pump inhibitors use and its association with premalignant gastric lesions: a systematic review and meta-analysis
Abstract
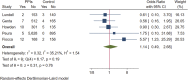
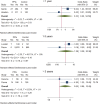
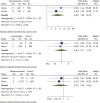
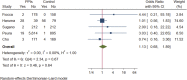
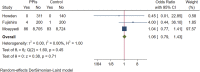
Background: Long-term maintenance therapy with proton pump inhibitors (PPIs) is a common treatment strategy for acid-related gastrointestinal diseases. However, concerns have been raised about the potential increased risk of gastric cancer and related precancerous lesions with long-term PPI use. This systematic review and meta-analysis aimed to evaluate this potential risk. Methods: We searched PubMed, Embase, and the Cochrane Central Register of Controlled Trials for randomised controlled trials published before 1 March 2023, with no language restrictions. The primary endpoint was the occurrence and progression of gastric mucosal atrophy, intestinal metaplasia, Enterochromaffin-like (ECL) cell hyperplasia, gastric polyps, and gastric cancer during the trial and follow-up. Data were analysed using a random effects model. Results: Of the 4,868 identified studies, 10 met the inclusion criteria and were included in our analysis, comprising 27,283 participants. Compared with other treatments, PPI maintenance therapy for more than 6 months was associated with an increased risk of ECL cell hyperplasia (OR 3.01; 95% CI 1.29 to 7.04; p = 0.01). However, no significant increase was found in the risk of gastric mucosal atrophy (OR 1.01; 95% CI 0.55 to 1.85; p = 0.97), intestinal metaplasia (OR 1.14; 95% CI 0.49 to 2.68; p = 0.76), gastric polyps (OR 1.13; 95% CI 0.68 to 1.89; p = 0.64), or gastric cancer (OR 1.06; 95% CI 0.79 to 1.43; p = 0.71). Conclusion: This systematic review and meta-analysis does not support an increased risk of gastric cancer or related precancerous lesions with long-term PPI maintenance therapy. However, long-term PPI use should be monitored for potential complications such as ECL cell hyperplasia. Further studies are needed to confirm these findings and evaluate the safety of PPI maintenance therapy for acid-related gastrointestinal diseases. Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, Identifier: PROSPERO (CRD42022379692).
Keywords: enterochromaffin-like cell; gastric cancer; gastric mucosal atrophy; gastric polyps; intestinal metaplasia; meta-analysis; proton pump inhibitors.
Copyright © 2023 Zheng, Lu and Song.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
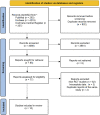
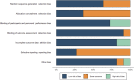
Figures



References
-
- Allemani C., Weir H. K., Carreira H., Harewood R., Spika D., Wang X. S., et al. (2015). Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet (London, Engl. 385 (9972), 977–1010. 10.1016/S0140-6736(14)62038-9 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

