URAT1 is expressed in cardiomyocytes and dotinurad attenuates the development of diet-induced metabolic heart disease
- PMID: 37694143
- PMCID: PMC10483053
- DOI: 10.1016/j.isci.2023.107730
URAT1 is expressed in cardiomyocytes and dotinurad attenuates the development of diet-induced metabolic heart disease
Abstract
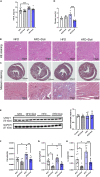
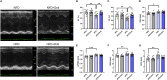
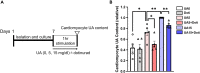
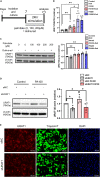
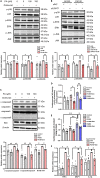
We recently reported that the selective inhibition of urate transporter-1 (URAT1), which is primarily expressed in the kidneys, ameliorates insulin resistance by attenuating hepatic steatosis and improving brown adipose tissue function in diet-induced obesity. In this study, we evaluated the effects of dotinurad, a URAT1-selective inhibitor, on the hearts of high-fat diet (HFD)-fed obese mice for 16-20 weeks and on neonatal rat cardiomyocytes (NRCMs) exposed to palmitic acid. Outside the kidneys, URAT1 was also expressed in cardiomyocytes and indeed worked as a uric acid transporter. Dotinurad substantially attenuated HFD-induced cardiac fibrosis, inflammatory responses, and cardiac dysfunction. Intriguingly, among various factors related to the pathophysiology of diet-induced obesity, palmitic acid significantly increased URAT1 expression in NRCMs and subsequently induced apoptosis, oxidative stress, and inflammatory responses via MAPK pathway, all of which were reduced by dotinurad. These results indicate that URAT1 is a potential therapeutic target for metabolic heart disease.
Keywords: Cell biology; Pathophysiology; Physiology.
© 2023 The Author(s).
Conflict of interest statement
Outside this study, Michihiro Yoshimura received research funds from Teijin Pharma Ltd., Shionogi & Co. Ltd., Otsuka Pharmaceutical Co. Ltd., and Mochida Pharmaceutical Co. Ltd., and speaker’s honorarium from Daiichi Sankyo Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Pfizer Japan Inc., AstraZeneca K.K., Otsuka Pharmaceutical Co. Ltd, Astellas Pharma Inc., Bayer Yakuhin Ltd., Novartis Pharma K.K., and Mochida Pharmaceutical Co., Ltd.
Figures


References
-
- Tanaka Y., Nagoshi T., Kawai M., Uno G., Ito S., Yoshii A., Kimura H., Inoue Y., Ogawa K., Tanaka T.D., et al. Close linkage between serum uric acid and cardiac dysfunction in patients with ischemic heart disease according to covariance structure analysis. Sci. Rep. 2017;7:2519. doi: 10.1038/s41598-017-02707-y. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

