Defining the proteomic landscape of cultured macrophages and their polarization continuum
- PMID: 37694300
- PMCID: PMC10953363
- DOI: 10.1111/imcb.12687
Defining the proteomic landscape of cultured macrophages and their polarization continuum
Abstract
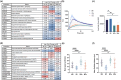
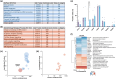
Macrophages have previously been characterized based on phenotypical and functional differences into suggested simplified subtypes of MØ, M1, M2a and M2c. These macrophage subtypes can be generated in a well-established primary monocyte culture model that produces cells expressing accepted subtype surface markers. To determine how these subtypes retain functional similarities and better understand their formation, we generated all four subtypes from the same donors. Comparative whole-cell proteomics confirmed that four distinct macrophage subtypes could be induced from the same donor material, with > 50% of 5435 identified proteins being significantly altered in abundance between subtypes. Functional assessment highlighted that these distinct protein expression profiles are primed to enable specific cell functions, indicating that this shifting proteome is predictive of meaningful changes in cell characteristics. Importantly, the 2552 proteins remained consistent in abundance across all macrophage subtypes examined, demonstrating maintenance of a stable core proteome that likely enables swift polarity changes. We next explored the cross-polarization capabilities of preactivated M1 macrophages treated with dexamethasone. Importantly, these treated cells undergo a partial repolarization toward the M2c surface markers but still retain the M1 functional phenotype. Our investigation of polarized macrophage subtypes therefore provides evidence of a sliding scale of macrophage functionality, with these data sets providing a valuable benchmark resource for further studies of macrophage polarity, with relevance for cell therapy development and drug discovery.
Keywords: dexamethasone; macrophage; macrophage subtype; polarization; proteomics.
© 2023 The Authors. Immunology & Cell Biology published by John Wiley & Sons Australia, Ltd on behalf of the Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The views expressed are those of the authors and not necessarily those of the National Health Service, NIHR or the Department of Health and Social Care.
Figures




References
-
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M‐1/M‐2 macrophages and the Th1/Th2 paradigm. J Immunol 2000; 164: 6166–6173. - PubMed
-
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1989; 7: 145–173. - PubMed
-
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004; 25: 677–686. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

